��Ŀ����
��11�֣�I�����������¼ƻ�����ָ��Τ���ڡ������ޡ����³ɹ�����˵���������ƻ�����û��һ���·����ļ����������ֳɵļ������ؼ������ۺϡ��ۺ�Ҳ�Ǵ��£���������ò������Ƴɵ�U�ιܡ�T�ιܡ��齺�ܡ����ɼ����ӳ���ͼ��ʾ��һ��װ�ã���U���м��������ĺ�ɫ��Һ�����ü�U����ѹ�ƣ����ǿɽ���Ӧ���ڶ���ʵ�飬�磺
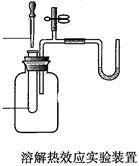
| A��֤�������е���������������ԭ��Ӧ��ʵ�飨�������⣩�� | B���ܽ���ЧӦʵ�飨�����������ˮ���� | C��װ�õ������Լ���ʵ�飻 | D������ijЩ�������ʵ�ʵ�飨��CO2��SO2��Cl2�������Һ�ķ�Ӧ���ȵȡ� |

���A��B��ѡ��һ��ʵ�飬������ʵ���װ��ͼ��д��ʵ�����ơ�Ҫ�ٳ�U����ѹ���⣬����ʵ����������Ʒ���ܴ�����������ѡȡ��
 ���ƿ��С�Թܡ���ͷ�ιܡ���Ƥ�����齺�ܡ����ܣ���ʵ��ҩƷ���Լ��Զ���������ͼ��ע����
���ƿ��С�Թܡ���ͷ�ιܡ���Ƥ�����齺�ܡ����ܣ���ʵ��ҩƷ���Լ��Զ���������ͼ��ע����
ʵ�����ƣ�
��
 �����ƿ����������;�治��ʵ������Zn�������ᷴӦ�Ƶõ�H2�����к�������ˮ�������⼰�����������������壬ijͬѧ������ֻ���ƿ��������¼���װ�ã���һ��˳�����ӣ���ﵽ�˵�����ͨ��ʱ��ÿһװ�ó�ȥһ�������Ŀ�ġ�
�����ƿ����������;�治��ʵ������Zn�������ᷴӦ�Ƶõ�H2�����к�������ˮ�������⼰�����������������壬ijͬѧ������ֻ���ƿ��������¼���װ�ã���һ��˳�����ӣ���ﵽ�˵�����ͨ��ʱ��ÿһװ�ó�ȥһ�������Ŀ�ġ�
��1���������ӵ�˳��Ϊ________________________________��
��2����ȥH2S�����ӷ���ʽΪ________________��
��3����ȥO
 2�����ӷ���ʽΪ________________���۲쵽������Ϊ________________��
2�����ӷ���ʽΪ________________���۲쵽������Ϊ________________��
��11�֣���4�֣���ͼ3�֣�ʵ������1�֣�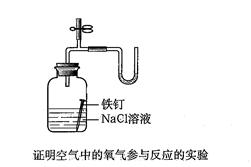
NH4NO3����
����װ��ֻ�軭����һ���ɣ�����������������֣�
�����һ��1�֣�����ÿ��2�֣�
��1��a��b��e��f��g��h��d��c
��2�� Cu2����H2S��CuS����2H��
Cu2����H2S��CuS����2H��
��3��4Fe2����O2��4H����4Fe3����2H2O
����װ��FeSO4��H2SO4��Һ�Ĺ��ƿ����Һ����ɫ��dz��ɫ����ػ�ɫ
����

��ϰ��ϵ�д�
�����Ŀ
 I�����������¼ƻ�����ָ��Τ���ڡ������ޡ����³ɹ�����˵���������ƻ�����û��һ���·����ļ����������ֳɵļ������ؼ������ۺϣ��ۺ�Ҳ�Ǵ��£���������ò������Ƴɵ�U�ιܡ�T�ιܡ��齺�ܡ����ɼ����ӳ���ͼ��ʾ��һ��װ�ã���U���м��������ĺ�ɫ��Һ�����ü�U����ѹ�ƣ����ǿɽ���Ӧ���ڶ���ʵ�飬�磺A��֤�������е���������������ԭ��Ӧ��ʵ�飨�������⣩��B���ܽ���ЧӦʵ�飨�����������ˮ����C��װ�õ������Լ���ʵ�飻D������ijЩ�������ʵ�ʵ�飨��CO2��SO2��Cl2�������Һ�ķ�Ӧ���ȵȣ����A��B��ѡ��һ��ʵ�飬������ʵ���װ��ͼ��д��ʵ�����ƣ�Ҫ�ٳ�U����ѹ���⣬����ʵ����������Ʒ���ܴ�����������ѡȡ�����ƿ��С�Թܡ���ͷ�ιܡ���Ƥ�����齺�ܡ����ܣ���ʵ��ҩƷ���Լ��Զ���������ͼ��ע����
I�����������¼ƻ�����ָ��Τ���ڡ������ޡ����³ɹ�����˵���������ƻ�����û��һ���·����ļ����������ֳɵļ������ؼ������ۺϣ��ۺ�Ҳ�Ǵ��£���������ò������Ƴɵ�U�ιܡ�T�ιܡ��齺�ܡ����ɼ����ӳ���ͼ��ʾ��һ��װ�ã���U���м��������ĺ�ɫ��Һ�����ü�U����ѹ�ƣ����ǿɽ���Ӧ���ڶ���ʵ�飬�磺A��֤�������е���������������ԭ��Ӧ��ʵ�飨�������⣩��B���ܽ���ЧӦʵ�飨�����������ˮ����C��װ�õ������Լ���ʵ�飻D������ijЩ�������ʵ�ʵ�飨��CO2��SO2��Cl2�������Һ�ķ�Ӧ���ȵȣ����A��B��ѡ��һ��ʵ�飬������ʵ���װ��ͼ��д��ʵ�����ƣ�Ҫ�ٳ�U����ѹ���⣬����ʵ����������Ʒ���ܴ�����������ѡȡ�����ƿ��С�Թܡ���ͷ�ιܡ���Ƥ�����齺�ܡ����ܣ���ʵ��ҩƷ���Լ��Զ���������ͼ��ע����








