��Ŀ����
2008�걱�����˻ᡰ���ơ�����õ��ǻ�����ȼ���飨C3H8����Ϥ����˻�������ȼ��Ϊ65%���飨C4H10����35%���飮��֪�����ȼ����Ϊ2221.5 kJ /mol,�����й�˵����ȷ����
A�����˻��ȼ����Ҫ�ǽ���ѧ����ת��Ϊ���ܺ���
B������ķе���������
C�����顢��������Ƭ�����ȼ�ϵ�أ��ڱ��鸽���IJ���Ϊ��ص�����
D������ȼ�յ��Ȼ�ѧ����ʽΪC3H8��g��+5O2��g��=3CO2��g��+4H2O��g�� ��H= ��2221.5kJ/mol
��ϰ��ϵ�д�
�����Ŀ


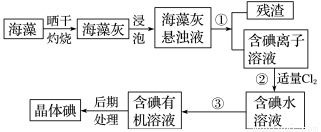
 C������D�������� e.�����ż� f.�ƾ���
C������D�������� e.�����ż� f.�ƾ���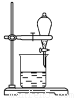
 ��ȴδ��Һ�����£�ԭ�������_________________��
��ȴδ��Һ�����£�ԭ�������_________________��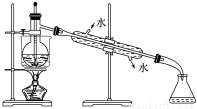
 HCl=2KCl+3Cl2��+7H2O+2CrCl3��Ӧ�С�
HCl=2KCl+3Cl2��+7H2O+2CrCl3��Ӧ�С�