��Ŀ����
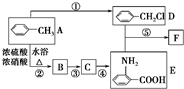
��֪����RNH2+R��CH2Cl
| һ������ |
����ͬϵ���ܱ����Ը��������Һ�������磺

��

����
 ��Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ���
��Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ����ش��������⣺
��1��C�Ľṹ��ʽ��


��2��D+E��F�Ļ�ѧ����ʽ


��3����֪E��һ��ͬ���칹�壨��λ��������һ�������£��ɾۺϳ��ȹ��Ժܺõĸ߷��ӣ�д���ϳɴ˸߷��ӻ�����Ļ�ѧ����ʽ


��4����Ӧ�١����У�����ȡ����Ӧ����
 ����ע����ǣ����ڱ����ױ�������������KMnO4��H+������-CH3��-COOH���Ƚ��У�CΪ
����ע����ǣ����ڱ����ױ�������������KMnO4��H+������-CH3��-COOH���Ƚ��У�CΪ ��D��E����ȡ����Ӧ������Ϣ���֪����FΪ
��D��E����ȡ����Ӧ������Ϣ���֪����FΪ ������л���Ľṹ�����ʺ���ĿҪ��ɽ����⣮
������л���Ľṹ�����ʺ���ĿҪ��ɽ����⣮ ����ע����ǣ����ڱ����ױ�������������KMnO4��H+������-CH3��-COOH���Ƚ��У�CΪ
����ע����ǣ����ڱ����ױ�������������KMnO4��H+������-CH3��-COOH���Ƚ��У�CΪ ��D��E����ȡ����Ӧ������Ϣ���֪����FΪ
��D��E����ȡ����Ӧ������Ϣ���֪����FΪ ��
����1�������Ϸ�����֪CΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��D��E����ȡ����Ӧ������Ϣ���֪����FΪ
 ����Ӧ�ķ���ʽΪ
����Ӧ�ķ���ʽΪ ��
���ʴ�Ϊ��
 ��
����3��E��һ��ͬ���칹�壨��λ��������һ�������£��ɾۺϳ��ȹ��Ժܺõĸ߷��ӣ����л���Ϊ

���ɷ������۷�Ӧ����Ӧ�ķ���ʽΪ
 ��
���ʴ�Ϊ��
 ��
����4����Ӧ�١����У���Ϊȡ����Ӧ����Ϊȡ����Ӧ����Ϊ������Ӧ����Ϊ��ԭ��Ӧ����Ϊȡ����Ӧ��
�ʴ�Ϊ���٢ڢݣ�

 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�(16��)���ݡ��й���ҩ��������������F�������Ʊ����ǵ䡱ҩƷ����������ʹ�����м�����ϳ�·��Ϊ��

![]() ��֪�� һ����������RNH2+
��֪�� һ����������RNH2+CH2Cl ��RNHCH2
+HCl��R��
������������
������ͬϵ���ܱ����Ը��������Һ�������磺
�����������������ԣ���������
��������Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ����
�ش��������⣺
��1��C�Ľṹ��ʽ�� ��
��2����д��D+E��F�Ļ�ѧ����ʽ�� ��
��3��E��һ�������£��ɾۺϳɺܺõĹ��ܸ߷��Ӳ��ϣ�д���ϳɴ˸߾���Ļ�ѧ����ʽ ��
��4����Ӧ��~���У�����ȡ����Ӧ���ǣ��Ӧ��ţ�
(5)��������������E��ͬ���칹�����Ŀ�ǣ� ����
����FeCl3��Һ����ɫ��Ӧ ���ܷ���������Ӧ �۱����ϵ�һ��ȡ����ֻ��3�֡�
A��3�� B��8�� C��10�� D��12��
(6) ��֪����ȩ��һ�������¿���ͨ��Perkin��Ӧ��������ᣨ����45~50%������Ӧ����ʽ���£�
C6H5CHO+ (CH3CO)2O �� C6H5CH=CHCOOH+CH3COOH
����ȩ �����
������ȩ�ı�������ȡ������Ҳ�ܷ���Perkin��Ӧ����Ӧ����IJ������£�
|
��Ӧ��
|
|
|
|
|
| ���ʣ�%�� | 15 | 23 | 33 | 0 |
|
��Ӧ��
|
|
|
|
|
| ���ʣ�%�� | 71 | 63 | 52 | 82 |
������ϱ��ش�ȡ������Perkin��Ӧ��Ӱ���У�д��3�����ɣ���
��
��
�� [��
(16��)���ݡ��й���ҩ��������������F�������Ʊ����ǵ䡱ҩƷ����������ʹ�����м�����ϳ�·��Ϊ��

 ��֪�� һ����������RNH2+
��֪�� һ����������RNH2+ CH2Cl ��
RNHCH2
CH2Cl ��
RNHCH2 +HCl��R��
+HCl��R�� ������������
������������
������ͬϵ���ܱ����Ը��������Һ�������磺
���� �������������ԣ���������
�������������ԣ���������
������ ��Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ����
��Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ����
�ش��������⣺
��1��C�Ľṹ��ʽ�� ��
��2����д��D+E��F�Ļ�ѧ����ʽ�� ��
��3��E��һ�������£��ɾۺϳɺܺõĹ��ܸ߷��Ӳ��ϣ�д���ϳɴ˸߾���Ļ�ѧ����ʽ ��
��4����Ӧ��~���У�����ȡ����Ӧ���ǣ��Ӧ��ţ�
(5)��������������E��ͬ���칹�����Ŀ�ǣ� ����
����FeCl3��Һ����ɫ��Ӧ ���ܷ���������Ӧ �۱����ϵ�һ��ȡ����ֻ��3�֡�
A��3�� B��8�� C��10�� D��12��
(6) ��֪����ȩ��һ�������¿���ͨ��Perkin��Ӧ��������ᣨ����45~50%������Ӧ����ʽ���£�
C6H5CHO + (CH3CO)2O �� C6H5CH=CHCOOH +CH3COOH
����ȩ �����
������ȩ�ı�������ȡ������Ҳ�ܷ���Perkin��Ӧ����Ӧ����IJ������£�
|
��Ӧ��
|
|
|
|
|
|
���ʣ�%�� |
15 |
23 |
33 |
0 |
|
��Ӧ��
|
|
|
|
|
|
���ʣ�%�� |
71 |
63 |
52 |
82 |
������ϱ��ش�ȡ������Perkin��Ӧ��Ӱ���У�д��3�����ɣ���
��
��
�� [��


 RNHCH2 R/+HCl��R��R/����������
RNHCH2 R/+HCl��R��R/����������
 ��Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ���
��Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ���


 RNHCH2 R/+HCl��R��R/����������
RNHCH2 R/+HCl��R��R/����������
 ��Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ���
��Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ���