��Ŀ����
(6��)��һ�������������Na2CO3��K2SO4��CuSO4��Ca(NO3)2��KCl��AgNO3�Ȼ�϶��ɣ�Ϊ�������ǣ���������ʵ�飺�ٽ�������������ˮ����������ɫ����Һ����������Һ�еμ�BaCl2��Һ���а�ɫ�������ɣ��۹��ˣ�����������������ϡ�����У����ֳ���ȫ���ܽ⡣���жϣ�
��1�����������п϶��� ���϶�û�� ������ �����û�ѧʽ��д��
��2��д��ʵ����з����ķ�Ӧ�����ӷ���ʽ�� ��
��1�����������п϶��� ���϶�û�� ������ �����û�ѧʽ��д��
��2��д��ʵ����з����ķ�Ӧ�����ӷ���ʽ�� ��
��1��Na2CO3����1�֣�K2SO4��CuSO4��Ca(NO3)2��AgNO3����2�֣�
KCl��1�֣���2��BaCO3+2H+==Ba2++H2O+CO2����2�֣�
KCl��1�֣���2��BaCO3+2H+==Ba2++H2O+CO2����2�֣�
��

��ϰ��ϵ�д�
�����Ŀ

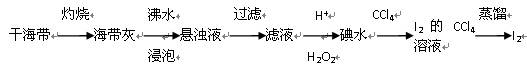
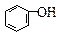 ��
�� ��FeCl3��Һ��
��FeCl3��Һ��