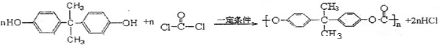��Ŀ����
��.2011��4��27��ij�й��ֲ̾��400�˴�װ�����겹��Ӫ���̷�1 401���������̷۱�����Ϊ�����������β������߳�����ֵ7.8��������ʳ�ÿ����°�����֪NaNO2�ܷ������·�Ӧ��2NaNO2��4HI===2NO����I2��2NaI��2H2O��
(1)������Ӧ����������_____������0.75 mol�Ļ�ԭ������������ԭ����������_____mol��
(2)����������Ӧ����������ֽ�������г��������ʽ���ʵ�飬�Լ���NaNO2��NaCl����ѡ�õ������У�������ˮ���ڵ��۵⻯����ֽ���۵��ۣ��ܰ��ǣ���ʳ�ף��ްơ�����ʵ��ʱ������ѡ�õ�������____________��
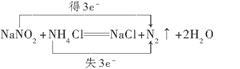
(3)ij����������Һ�к���2%��5%��NaNO2��ֱ���ŷŻ������Ⱦ�������Լ���_____(�����)��ʹNaNO2ת��Ϊ�����������Ⱦ��N2��Ӧ�Ļ�ѧ����ʽΪ________________________ (���������ת�Ƶ���Ŀ�ͷ���)��
��NaCl����NH4Cl����H2O2����ŨH2SO4
(4)������������Һ��һ���ܴ����������________������������������������������������
A��NH ��Cl����OH����CO
��Cl����OH����CO B��Fe2����NO3-��Cl����H��
B��Fe2����NO3-��Cl����H��
C��CH3COO����NH ��H����Cl�� D��CH3COO����Na����Cl����SO
��H����Cl�� D��CH3COO����Na����Cl����SO
��.ʵ����Ϊ�������й������ĺ�������������Ϳ��CuI����ֽ��������ֽ�Ƿ��ɫ����ɫ�����仯����ȥ��ʱ�����жϿ����еĺ��������䷴ӦʽΪ
4CuI��Hg===Cu2HgI4��2Cu��
(1)������Ӧ����Cu2HgI4�У�CuԪ����________�ۡ�
(2) CuI����Cu2����I��ֱ�ӷ�Ӧ�Ƶã�����ƽ���з�Ӧ�����ӷ���ʽ��
 Cu2����
Cu2���� I��===
I��=== CuI��
CuI�� I
I
��(1)NaNO2��0.75��(2)�ڢݡ�
(3)�� (4)D
(4)D
��.(1)��1��(2) 2Cu2����5I��===2CuI��I3��
��������
�����������1�����ݷ�Ӧ�ķ���ʽ��֪�����������е�Ԫ�صĻ��ϼ۴ӣ�3�۽��͵���2�ۣ��õ�1�����ӣ��������������ǻ�ԭ����HI��IԪ�صĻ��ϼ۴ӣ�1�����ߵ�0�ۣ�ʧȥ1�����ӣ�����ݵ��ӵ�ʧ�غ��֪������0.75 mol�Ļ�ԭ������������ԭ����������0.75mol��
��2�����ݷ�Ӧ�ķ���ʽ��֪���÷�Ӧ�������������½��еģ����Ա���ѡ�õ������е��۵⻯����ֽ��ʳ�ף���ѡ�ڢݡ�
��3��ʹNaNO2ת��Ϊ�����������Ⱦ��N2����˵���ڷ�Ӧ���������������������õ����ӣ�����ѡ���Ӧ���ǻ�ԭ����NH4���еĵ�Ԫ�ش�����ͼ�̬�����л�ԭ�ԣ�����ѡ����Լ����Ȼ�泥�����ѡ�ڣ���Ӧ�ķ���ʽ�� ��
��
��4��A�е�OH����NH4�����ܴ������棻B����Һ�����ԣ���NO3��������Fe2�����ܴ������棻C�е�CH3COO����H�����ܴ������棬���Դ�ѡD��
��.��1����Cu2HgI4�У�Hg�ԣ�2�ۣ�I�ǣ�1�ۣ�����ͭ�ǣ�1�ۡ�
��2���ڷ�Ӧ��ͭ�Ļ��ϼ۴ӣ�2�۽��͵���1�ۣ��õ�1�����ӣ�����Ԫ�ص⻯�ϼ۴ӣ�1�����ߵ�0�ۣ�ʧȥ1�����ӣ�����ݵ��ӵĵ�ʧ�غ��֪����ƽ��ķ���ʽӦ����2Cu2����5I��===2CuI��I3����
���㣺����������ԭ��Ӧ���й��жϡ����㡢��ƽ�Լ����ӹ�����ж�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ����ע�ض�ѧ������֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ�����������������ͷ�����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������ͷ�ɢ˼ά����������Ĺؼ���ȷ����й�Ԫ�صĻ��ϼ۱仯�����Ȼ�������йصĸ������ϵ��ӵ�ʧ�غ������ʽ������жϼ��ɡ�

��.2011��4��27��ij�й��ֲ̾��400�˴�װ�����겹��Ӫ���̷�1 401���������̷۱�����Ϊ�����������β������߳�����ֵ7.8��������ʳ�ÿ����°�����֪NaNO2�ܷ������·�Ӧ��2NaNO2��4HI===2NO����I2��2NaI��2H2O��
(1)������Ӧ����������_____������0.75 mol�Ļ�ԭ������������ԭ����������_____mol��
(2)����������Ӧ����������ֽ�������г��������ʽ���ʵ�飬�Լ���NaNO2��NaCl����ѡ�õ������У�������ˮ���ڵ��۵⻯����ֽ���۵��ۣ��ܰ��ǣ���ʳ�ף��ްơ�����ʵ��ʱ������ѡ�õ�������____________��
(3)ij����������Һ�к���2%��5%��NaNO2��ֱ���ŷŻ������Ⱦ�������Լ���_____(�����)��ʹNaNO2ת��Ϊ�����������Ⱦ��N2��Ӧ�Ļ�ѧ����ʽΪ________________________ (���������ת�Ƶ���Ŀ�ͷ���)��
��NaCl����NH4Cl����H2O2����ŨH2SO4
(4)������������Һ��һ���ܴ����������________������������������������������������
A��NH ��Cl����OH����CO ��Cl����OH����CO | B��Fe2����NO3-��Cl����H�� |
C��CH3COO����NH ��H����Cl�� ��H����Cl�� | D��CH3COO����Na����Cl����SO |
4CuI��Hg===Cu2HgI4��2Cu��
(1)������Ӧ����Cu2HgI4�У�CuԪ����________�ۡ�
(2) CuI����Cu2����I��ֱ�ӷ�Ӧ�Ƶã�����ƽ���з�Ӧ�����ӷ���ʽ��
 Cu2����
Cu2���� I��===
I��=== CuI��
CuI�� I
I
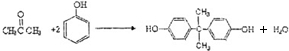
˫��A��Ҳ��BPA�����������������ϵ�ԭ�ϡ�ŷ����Ϊ��˫��A��ƿ���շ������죬��2011��3��2���𣬽�ֹ��������ѧ����˫��A��Ӥ����ƿ��
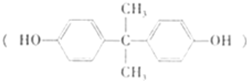
��1��˫��A�Ľṹ��ʽ��ͼ����ʾ��д��˫��A�ķ���ʽ 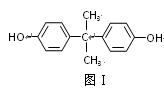
��2��˫��A�ɱ��Ӻͱ�ͪ�� 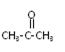 �������Խ����з�Ӧ�Ƶá�д����Ӧ�Ļ�ѧ����ʽ
�������Խ����з�Ӧ�Ƶá�д����Ӧ�Ļ�ѧ����ʽ
��3����˫��A������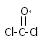 ��Ϊԭ�Ϻϳ�һ�ַ������ϡ�����̼���������PC
��Ϊԭ�Ϻϳ�һ�ַ������ϡ�����̼���������PC
�ṹ��ʽ��ͼ����PCΪԭ���Ƴɵ�Ӥ����ƿ��ʹ�ù����л����ų���������˫��A������ʹ�ÿ�����Ӥ���Ľ�����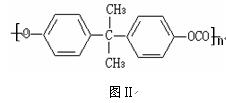
�� ��˫��A�����ϳ�PC�ķ�Ӧ�� ��Ӧ
��д����PC���ų�˫��A�Ļ�ѧ����ʽ
(4)���й���˫��A��PC�������У���ȷ���� �� ˫ѡ ��
| A��˫��A���������е�̼ԭ�Ӷ���ͬһƽ���� |
| B��˫��A��PC���Ƿ��������� |
| C��һ��������˫��A��PC�����Ժ�NaOH��Һ��Ӧ |
| D��һ��������1mol PC���Ժ�6mol H2�����ӳɷ�Ӧ |
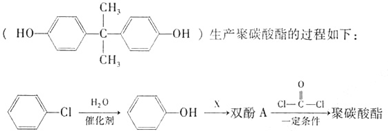
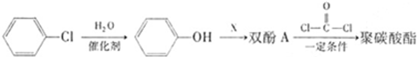
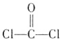 �����ʵ���֮��Ϊ1��1��д�����ɾ�̼�����Ļ�ѧ����ʽ
�����ʵ���֮��Ϊ1��1��д�����ɾ�̼�����Ļ�ѧ����ʽ