��Ŀ����
���к��ȡ���һ���Ȼ�ѧ�е���Ҫ������к��ȡ��Ķ���Ϊ����ϡ��Һ�У��������кͷ�Ӧ����1mol H2Oʱ�ķ�Ӧ�Ƚ����к��ȡ����¹����к��ȵ�������ȷ����
| A����ϡ��Һ��������ͼӦ���к�����ֵ����� |
| B����ϡ��Һ��1mol���1mol�Ӧ�ų������������ |
| C����ϡ��Һ��HCl��NaOH��Ӧ���к�����HNO3��KOH��Ӧ���к�����ֵ��� |
| D����Ũ�����������������Һ�иպ�����1molˮʱ��������������Ϊ�к��� |
C
�����к��ȵĸ���
�ᡢ���Ԫ����ǿ������Һ��Ũ�ȵȾ���Ӱ���кͷ�Ӧ���ų������������ų�AB��C�������⣻
D����Ũ������������ˮ��Һ��ϵĹ����л�ų��������ȣ��������к��ȣ��ų�
�ᡢ���Ԫ����ǿ������Һ��Ũ�ȵȾ���Ӱ���кͷ�Ӧ���ų������������ų�AB��C�������⣻
D����Ũ������������ˮ��Һ��ϵĹ����л�ų��������ȣ��������к��ȣ��ų�

��ϰ��ϵ�д�
�����Ŀ
 ����Ӧ���������ų��������ɼ����к��ȡ��Իش��������⡣
����Ӧ���������ų��������ɼ����к��ȡ��Իش��������⡣
 ��H= ��99kJ��mol-1����ش��������⣺
��H= ��99kJ��mol-1����ش��������⣺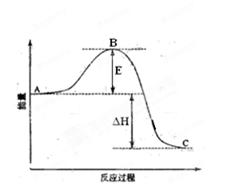
 ��1��ͼ��A��C�ֱ��ʾ ��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ��
��1��ͼ��A��C�ֱ��ʾ ��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ�� C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������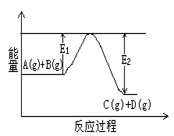
 O���Ȼ�ѧ����ʽΪ ��
O���Ȼ�ѧ����ʽΪ �� ����Һ̬˫��ˮ��H2O2����
����Һ̬˫��ˮ��H2O2����