��Ŀ����
����10�֣���ϩ��(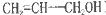 )��һ����ɫ�д̼�����ζ��Һ�壬����Ҫ���л��ϳ�ԭ�ϡ���ش�
)��һ����ɫ�д̼�����ζ��Һ�壬����Ҫ���л��ϳ�ԭ�ϡ���ش�
��1����ϩ���ķ���ʽΪ__________����ϩ���к��еĹ����ŵ�������__________��
��2��0.3mol��ϩ�������������Ʒ�Ӧ�������ɱ�״���µ�����_________ L��
��3��д����ϩ������ˮ��Ӧ�Ļ�ѧ����ʽ____________________________
��Ӧ����Ϊ____________________
��4����ϩ���� ����������Ӧ�Ļ�����ʽΪ��________________________________������Ӧ���ɵIJ�����һ�������¿��Է����Ӿ۷�Ӧ�õ��߷��ӻ������ṹ��ʽΪ____________________________��
����������Ӧ�Ļ�����ʽΪ��________________________________������Ӧ���ɵIJ�����һ�������¿��Է����Ӿ۷�Ӧ�õ��߷��ӻ������ṹ��ʽΪ____________________________��
��1��C3H6O��̼̼˫�����ǻ���
��2��3.36
��3��CH2=CH-CH2OH+Br2��CH2BrCHBrCH2OH���ӳɷ�Ӧ��
��4��CH2=CH-CH2OH+ CH3CO18OH CH3COOCH2CH=CH2+H218O��
CH3COOCH2CH=CH2+H218O��
���������������1���ɱ�ϩ���Ľṹ��ʽ�ж������ʽΪC3H6O����ϩ���к��еĹ����ŵ�������̼̼˫�����ǻ���
��2����ϩ�������д���1���ǻ�������1mol��ϩ����Na��Ӧ����0.5mol��������0.3mol�ı�ϩ����Na��Ӧ����0.15mol���������ڱ�״���µ������0.15mol��22.4L/mol=3.36L��
��3����ϩ�������е�̼̼˫�����巢���ӳɷ�Ӧ������CH2BrCHBrCH2OH����ѧ����ʽΪCH2=CH-CH2OH+Br2��CH2BrCHBrCH2OH��
��4����ϩ�������е��ǻ������ᷢ��������Ӧ������������Ӧ�ķ�Ӧԭ���������е��ǻ��봼�����е���ԭ�ӽ������ˮ������O-18��ˮ�����У���ѧ����ʽΪCH2=CH-CH2OH+ CH3CO18OH CH3COOCH2CH=CH2+H218O�����ɵ��л������к���̼̼˫�������Է����Ӿ۷�Ӧ�����ɵIJ���Ľṹ��ʽΪ
CH3COOCH2CH=CH2+H218O�����ɵ��л������к���̼̼˫�������Է����Ӿ۷�Ӧ�����ɵIJ���Ľṹ��ʽΪ ��
��
���㣺�����л������ʽ���жϣ������ŵ����ơ�����Ӧ�ã���ѧ����ʽ����д

���и��ֱ仯�У������ڻ�ѧ�仯����
| A������Һ�е��뱥����������Һ��������ɫ���� |
| B�����ȵ����õ���ɫ����ˮ����ͭ��ĩ |
| C�����ˮ�е��뱥���Ȼ�����Һ����ȡ������������ |
| D��������Һ�е�������Ǧ��Һ��������ɫ���� |
1-������������Ũ���������£�ͨ��������Ӧ�Ƶ����ᶡ������Ӧ�¶�Ϊ115�桫125�棬���������������
| A��������ˮԡ���� |
| B����������ᶡ���⣬���ܻ����������� |
| C���ᴿ���ᶡ����Ҫ����ˮ������������Һϴ�� |
| D�������������������1������ת���� |
���л��ﻯѧ������
���ǻ�����ȩ���׳�PHBA����-����Ҫ���л�����ԭ�ϡ���ṹ��ͼ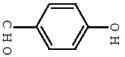 ��ʾ������������ԶԼ�����Ϊԭ�Ϻϳ�PHBA��;�����£�
��ʾ������������ԶԼ�����Ϊԭ�Ϻϳ�PHBA��;�����£�
��1��PHBA�Ĺ����ŵ�����Ϊ_______��
��2�����й�PHBA��˵����ȷ����_______��
| A��PHBA�ķ���ʽΪC7H6O2 |
| B��PHBA��һ�ַ����� |
| C��1mol PHBA�������4mo1 H2��Ӧ |
| D��PHBA����NaHCO3��Һ��Ӧ����CO2 |
��4����Ӧ�۵Ļ�ѧ����ʽΪ_______��
��5���úϳ�;���еķ�Ӧ�٢ݵ�����Ϊ_______��
��6��E�ж���ͬ���칹�壬������������������ͬ���칹��Ľṹ��ʽΪ______ ��ֻдһ�֣���
a�������ϴ���2�ֲ�ͬ��ѧ��������ԭ�ӣ��Ҹ�����Ϊ1:2
b����FeCl3��Һ��ʾ������ɫ
c����ʹ������Ȼ�̼��Һ��ɫ


