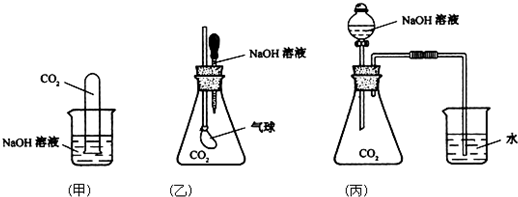
��Ŀ����
Ϊ̽��CO2�������NaOH��Һȷʵ�����˻�ѧ��Ӧ���ס��ҡ�����λͬѧ�������������ʵ��װ�ã���ش��������⣺
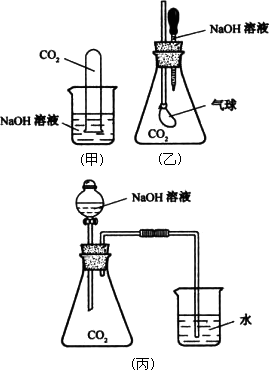
(1)��д��CO2�������NaOH������Ӧ�����ӷ���ʽ��________��
(2)ѡ������һ��ʵ��װ�ã�����Ԥ�Ƴ��ֵ�ʵ�������Ͳ�����ʵ�������ԭ��
��ѡ���ʵ��װ����________��ʵ��������________�����Ͳ�����ʵ�������ԭ��________��
(3)�ס��ҡ���ͬѧ��Ƶ����������У���һ��������ʵ�ʲ����п����ԺͰ�ȫ�Դ������⣬�÷�����________��(��ס��һ��)
(4)�����һ��ʵ��������ɵIJ����к���Na2CO3��(���������Լ���ʵ������ͽ��ۣ�)
___________________��
�𰸣�

��ϰ��ϵ�д�
 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
�����Ŀ