��Ŀ����
��2010?��ͨģ�⣩�����Ṥ�յ�β������ˮ��ʯ��ʯ����̿��̼����狀�KClΪԭ�Ͽ��Ժϳ�����ҪӦ�ü�ֵ���ơ�����ء���������淋����ʣ��ϳ�·�����£�
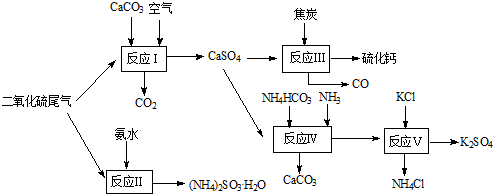
��1�����������У���Ӧ���������������������д���÷�Ӧ���ܷ���ʽ
��2����Ӧ������Ҫ����Һ�м��������ĶԱ����ӵ����ʣ�����ܵ�������
��3����Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ
��4����Ӧ����ӦҺ��40%�Ҷ�����Һ���¶ȿ�����25�棬����صIJ��ʳ���90%����ԭ����
��5�������ڲ��β����SO2��������
A�����з�̪��NaOH��Һ B������KMnO4
C�����е��۵ĵ�ˮ D��BaCl2��Һ��

��1�����������У���Ӧ���������������������д���÷�Ӧ���ܷ���ʽ
2CaCO3+2SO2+O2�T2CaSO4+2CO2
2CaCO3+2SO2+O2�T2CaSO4+2CO2
����2����Ӧ������Ҫ����Һ�м��������ĶԱ����ӵ����ʣ�����ܵ�������
��ֹ������隣�����
��ֹ������隣�����
����3����Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ
1��4
1��4
����4����Ӧ����ӦҺ��40%�Ҷ�����Һ���¶ȿ�����25�棬����صIJ��ʳ���90%����ԭ����
�Ҷ������л��ܼ��ܼ�СK2SO4���ܽ�ȣ�ʹ����س������
�Ҷ������л��ܼ��ܼ�СK2SO4���ܽ�ȣ�ʹ����س������
����5�������ڲ��β����SO2��������
BC
BC
��A�����з�̪��NaOH��Һ B������KMnO4
C�����е��۵ĵ�ˮ D��BaCl2��Һ��
��������1�����ݷ�Ӧ���������д����Ӧ�Ļ�ѧ����ʽ��
��2�������ᰱ�е���Ϊ+4�ۣ��ױ������е�����������
��3������Ԫ�صĻ��ϼ۱仯����������������ԭ�������û�ѧ��Ӧ����ʽ�������������뻹ԭ�������ʵ���֮�ȣ�
��4������������ڲ�ͬ�ܼ����ܽ�ȵIJ�ͬ���ﵽ���������Ŀ�ģ�
��5������KMnO4������������������ɫ��KMnO4��Һ��ɫ���������������������ԭ��Ӧ��������������ɫ��
��2�������ᰱ�е���Ϊ+4�ۣ��ױ������е�����������
��3������Ԫ�صĻ��ϼ۱仯����������������ԭ�������û�ѧ��Ӧ����ʽ�������������뻹ԭ�������ʵ���֮�ȣ�
��4������������ڲ�ͬ�ܼ����ܽ�ȵIJ�ͬ���ﵽ���������Ŀ�ģ�
��5������KMnO4������������������ɫ��KMnO4��Һ��ɫ���������������������ԭ��Ӧ��������������ɫ��
����⣺��1��̼��ơ����������������Ӧ��������ƺͶ�����̼������ʽΪ2CaCO3+2SO2+O2�T2CaSO4+2CO2���ʴ�Ϊ��2CaCO3+2SO2+O2�T2CaSO4+2CO2��
��2���Ա����Ӿ��л�ԭ�ԣ������е��������������ԣ������ᰱ��+4�۵�����л�ԭ�ԣ��ʴ�Ϊ����ֹ������隣�������
��3����Ӧ��ѧ����ʽΪ��CaSO4+4C=CaS+4CO����������ΪCaSO4����ԭ��ΪC���������뻹ԭ�����ʵ���֮��Ϊ1��4���ʴ�Ϊ��1��4��
��4����ӦV��ѡ����40%���Ҷ�����Һ���¶ȿ�����25�棬��ʱ����صIJ��ʳ���90%��ѡ��40%���Ҷ�����Һԭ���������Ҷ�������������ܽ�ȣ�������������
�ʴ�Ϊ���Ҷ������л��ܼ��ܼ�СK2SO4���ܽ�ȣ�ʹ����س��������
��5������KMnO4���������Ӧ�ķ���ʽΪ��5SO2+2MnO4-+2H2O=2Mn2++4H++5SO42-���������������������ԭ�ķ���ʽΪ��SO2+I2+2H2O=H2SO4+2HI���������꣬������û�еⵥ�ʲ�������ɫ���ʴ�Ϊ��BC��
��2���Ա����Ӿ��л�ԭ�ԣ������е��������������ԣ������ᰱ��+4�۵�����л�ԭ�ԣ��ʴ�Ϊ����ֹ������隣�������
��3����Ӧ��ѧ����ʽΪ��CaSO4+4C=CaS+4CO����������ΪCaSO4����ԭ��ΪC���������뻹ԭ�����ʵ���֮��Ϊ1��4���ʴ�Ϊ��1��4��
��4����ӦV��ѡ����40%���Ҷ�����Һ���¶ȿ�����25�棬��ʱ����صIJ��ʳ���90%��ѡ��40%���Ҷ�����Һԭ���������Ҷ�������������ܽ�ȣ�������������
�ʴ�Ϊ���Ҷ������л��ܼ��ܼ�СK2SO4���ܽ�ȣ�ʹ����س��������
��5������KMnO4���������Ӧ�ķ���ʽΪ��5SO2+2MnO4-+2H2O=2Mn2++4H++5SO42-���������������������ԭ�ķ���ʽΪ��SO2+I2+2H2O=H2SO4+2HI���������꣬������û�еⵥ�ʲ�������ɫ���ʴ�Ϊ��BC��
���������⿼�������ʵ��Ʊ����̷�������Ӧ�����жϣ���Ӧ������ѡ���ԭ����ϸ�������������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д�
�����Ŀ
