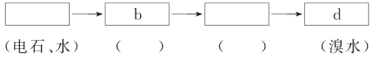
��Ŀ����
����Ŀ����֪��2CO(g)+O2(g)��2CO2(g) ��H=��565.2 kJ��mol��1������˵������ȷ����
A��CO(g)+![]() O2(g)��CO2(g) ��H=��282.6 kJ��mol��1
O2(g)��CO2(g) ��H=��282.6 kJ��mol��1
B��2mol CO(g)��1mol O2(g)��Ӧ����2mol CO2(s)�ų�����С��565.2 kJ
C����ͬ��������2mol CO(g)��1mol O2(g)������������2mol CO2(g)��������
D����2mol CO��1mol O2�Ļ�ѧ�����������������γ�2mol CO2��ѧ�����ų�������
���𰸡�B
��������
���������A����Ӧ�ȵĴ�С�����ʵ�����������������1molһ����̼ȼ�շų�������Ϊ��282.6kJ����Ӧ�ȵĻ�ѧ����ʽΪCO(g)+![]() O2(g)�TCO2(g)��H=-282.6kJmol��1����A��ȷ��B�������ɹ���Ҫ�ų�����������2molCO(g)��1molO2(g)��Ӧ����2molCO2(s)�ų���������565.2kJ����B����C������Ӧ�Ƿ��ȷ�Ӧ���Է�Ӧ��������������������������������2molCO(g)��1molO2(g)������������2molCO2(g)������������C��ȷ��D�����ݻ�ѧ��Ӧ��ʵ�ʣ���Ϊ��Ӧ���������������������������÷�ӦΪ���ȷ�Ӧ�����Ӧ�����м������յ�������С���γ����������ų�������������D��ȷ����ѡB��
O2(g)�TCO2(g)��H=-282.6kJmol��1����A��ȷ��B�������ɹ���Ҫ�ų�����������2molCO(g)��1molO2(g)��Ӧ����2molCO2(s)�ų���������565.2kJ����B����C������Ӧ�Ƿ��ȷ�Ӧ���Է�Ӧ��������������������������������2molCO(g)��1molO2(g)������������2molCO2(g)������������C��ȷ��D�����ݻ�ѧ��Ӧ��ʵ�ʣ���Ϊ��Ӧ���������������������������÷�ӦΪ���ȷ�Ӧ�����Ӧ�����м������յ�������С���γ����������ų�������������D��ȷ����ѡB��