��Ŀ����
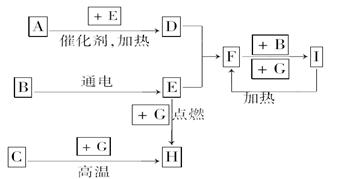
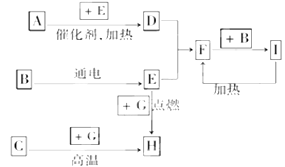
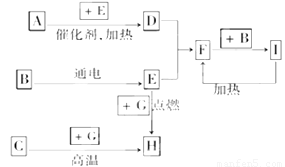
��������ѧ��ѧ�г������ʼ�ķ�Ӧת����ϵͼ�����в��ֲ�������ȥ�������£�GΪ���嵥�ʣ�B��IΪҺ�壬���Ϊ���壮AΪ�����I��Ũ��Һ��G�ڼ�������������F��B��C.H��������ҵ��ұ�������Ļ�ԭ�����밴Ҫ�����
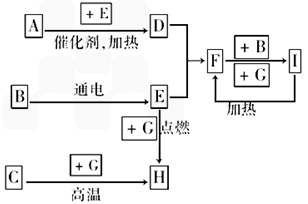
(1)д���������ʵĻ�ѧʽ�� A��________��B��________��C��________
(2)д��F��B��I�Ļ�ѧ����ʽ________________________________��
(3)д��G��I��Ũ��Һ��Ӧ�Ļ�ѧ����ʽ______________________________________��
(2)д��F��B��I�Ļ�ѧ����ʽ________________________________��
(3)д��G��I��Ũ��Һ��Ӧ�Ļ�ѧ����ʽ______________________________________��
(1)NH3��H2O��CO2
(2)3NO2��H2O==2HNO3��NO��
(3)C��4HNO3(Ũ)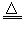 CO2����4NO2����2H2O
CO2����4NO2����2H2O
(2)3NO2��H2O==2HNO3��NO��
(3)C��4HNO3(Ũ)
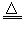 CO2����4NO2����2H2O
CO2����4NO2����2H2O
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ