��Ŀ����
��֪��SO2(g)�� O2(g)
O2(g) SO3(g)����H����98 kJ/mol��ij�¶��£���һ���Ϊ2 L���ܱ������г���0.2 mol SO2��0.1 mol O2,5 min��ﵽƽ�⣬���ų�����11.76 kJ������˵����ȷ����(����)
SO3(g)����H����98 kJ/mol��ij�¶��£���һ���Ϊ2 L���ܱ������г���0.2 mol SO2��0.1 mol O2,5 min��ﵽƽ�⣬���ų�����11.76 kJ������˵����ȷ����(����)
| A��5 min����O2��ʾ�ķ�Ӧ����Ϊ0.12 mol/(L��min) |
| B���÷�Ӧ��ƽ�ⳣ����ֵΪ7.5 |
| C��SO2��ƽ��ת����Ϊ60% |
| D�������������ʹ��H��С |
C
����

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д���֪��Ӧ ��H<O������˵����ȷ��( )��
��H<O������˵����ȷ��( )��
| A�������¶ȣ�����Ӧ�������ӣ�����Ӧ���ʼ�С |
| B�������¶������ڷ�Ӧ�������ӣ��Ӷ����̴ﵽƽ���ʱ�� |
| C���ﵽƽ��������¶Ȼ�����ѹǿ�������ڸ÷�Ӧƽ�������ƶ� |
| D���ﵽƽ������¶Ȼ��Сѹǿ�������ڸ÷�Ӧƽ�������ƶ� |
���淴Ӧ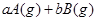
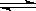
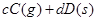
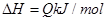 ����Ӧ�����У���������������ʱ��C�ڻ�����еĺ������¶ȣ�T���Ĺ�ϵ��ͼI��ʾ����Ӧ���ʣ�v����ѹǿ��p���Ĺ�ϵ��ͼII��ʾ����ͼ����������˵����ȷ���ǣ�������
����Ӧ�����У���������������ʱ��C�ڻ�����еĺ������¶ȣ�T���Ĺ�ϵ��ͼI��ʾ����Ӧ���ʣ�v����ѹǿ��p���Ĺ�ϵ��ͼII��ʾ����ͼ����������˵����ȷ���ǣ�������
| A��T1��T2��Q��0 |
| B������ѹǿ������B��ת�������� |
| C����Ӧ��ƽ�������������D��ƽ�������ƶ��� |
| D��a+b��c+d |
����������ʢ�й���ϡ������Թ��У���Ӱ�������������ʵ�������(����)��
| A�������Ũ�� | B�������ı���� | C����Һ���¶� | D��������Na2SO4 |
���й������ʡ���Ӧ�̶ȡ���˵����ȷ���ǣ� ��
| A��һ�������£�2molsO2������O2��Ӧ�ɵõ�2molsO3 |
| B����4mol HCl��Ũ������������MnO2���ȷ�Ӧ���Ʊ�1mol Cl2 |
| C��10mL 18.0mol/L H2SO4������ͭ���ȷ�Ӧ���Ʊ�0.09molsO2 |
| D��һ�������£�1mol N2��3mol H2��Ӧ���Ʊ�1.0mol NH3 |
��һ�������µĺ����ܱ������з�����Ӧ��CO2��g����3H2��g�� CH3OH��g����H2O��g����ͼ1��ʾ��Ӧ�����������ı仯��ͼ2��ʾ��Ӧ����������Ũ�ȵı仯�������й�˵����ȷ���ǣ�������
CH3OH��g����H2O��g����ͼ1��ʾ��Ӧ�����������ı仯��ͼ2��ʾ��Ӧ����������Ũ�ȵı仯�������й�˵����ȷ���ǣ�������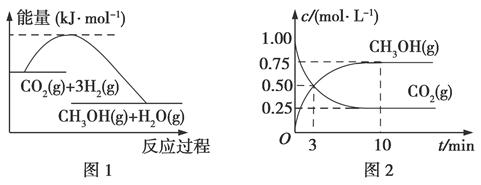
| A���÷�Ӧ���ʱ���ر䣺��H��0����S��0 |
| B���¶Ƚ��ͣ��÷�Ӧ��ƽ�ⳣ��K���� |
| C�������¶ȣ�n��CH3OH��/n��CO2������ |
| D���ӷ�Ӧ��ʼ��ƽ�⣬��������ʾ��ƽ����Ӧ����Ϊ2.25 mol/��L��min�� |
���ݻ��㶨���ܱ������У�һ������SO2��1.1 mol O2������Ӧ��2SO2(g)��O2(g) 2SO3(g)����H��0������������ʵ�������0.315 molʱ����Ӧ�ﵽƽ�⣬SO2��ƽ��ת������90%������˵����ȷ���ǣ� ��
2SO3(g)����H��0������������ʵ�������0.315 molʱ����Ӧ�ﵽƽ�⣬SO2��ƽ��ת������90%������˵����ȷ���ǣ� ��
| A����ͬ�����£�ƽ��ʱ������ϡ�����壬SO2��ת�������� |
| B����Ӧ��ʼʱ����������ͨ���SO2�����ʵ�����0.7 mol |
| C�����������������䣬�������¶ȣ�����Ӧ���ʼ�С�̶ȱ��淴Ӧ���ʼ�С�̶ȴ� |
| D�����������������䣬����С�����������Ӧ�ﵽƽ��ʱ��������1.485 mol���� |
�ں����µ��ܱ�������,�п��淴Ӧ:2NO2 N2O4,���в���˵����Ӧ�ﵽ��ƽ��״̬���� (����)
N2O4,���в���˵����Ӧ�ﵽ��ƽ��״̬���� (����)
| A��N2O4����������N2O4�ֽ��������ʱ |
| B���������ƽ����Է����������ֲ���ʱ |
| C��NO2�ķ�������N2O4��������Ϊ2��1ʱ |
| D����ϵ��ɫ���ٷ����ı�ʱ |
 2C(g) ��H<0���ﵽƽ��ı�һ��������������(Y)�ı仯������ͼ�����ߵ���( )
2C(g) ��H<0���ﵽƽ��ı�һ��������������(Y)�ı仯������ͼ�����ߵ���( )