��Ŀ����
(18��)�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
(1)�ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ����________________��
(2)�ڡ��ߵ���ۺ��������������ǿ�����ģ���ԭ�ӽṹ����ԭ��
__________��ԭ�Ӱ뾶�����õ��������������ǽ�����������
(3)�١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д������һ�ֻ�����ĵ���ʽ_______________��
(4)�ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬�������ʲ������÷�Ӧ��������(�����)________��
a��MnO2����b�� CuSO4 c��Na2SO3 d��FeCl3
(5) W��������ڵ�ͬ����Ԫ�ء����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
| ��� | ���� | ��ѧ����ʽ |
| ʾ�� | ������ | H2WO3��3H3PO3===3H3PO4��H2W�� |
| 1 | | |
| 2 | | |

X��Һ��Y��Һ��Ӧ�����ӷ���ʽ______________��
N���ĵ��ʵĻ�ѧ����ʽΪ____________��
M��Һ������Ũ���ɴ�С������˳����______________ ��
M�������ӵļ������� __________��
(18��)
��1���������ڣ���IVA�� -------------------------------------------------------------1��
��2��ͬһ����Ԫ�ش��ϵ���ԭ�Ӻ�����Ӳ�����������----------------------------1��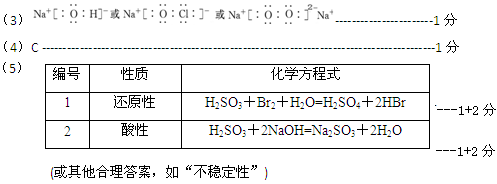
��6��Al3����3NH3��H2O===Al(OH)3����3NH4+ -------------------------------------------2��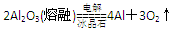 -------------------------------------------------------2��
-------------------------------------------------------2��
c(Cl-)>c(NH4+)>c(H+)>c(OH-)��c(NO3-)>c(NH4+)>c(H+)>c(OH-) ------------2��
ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠�����-------------------------------------------------2��
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�(18��)�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
(1)�ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ����________________��
(2)�ڡ��ߵ���ۺ��������������ǿ�����ģ���ԭ�ӽṹ����ԭ��
__________��ԭ�Ӱ뾶�����õ��������������ǽ�����������
(3)�١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д������һ�ֻ�����ĵ���ʽ_______________��
(4)�ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬�������ʲ������÷�Ӧ��������(�����)________��
a��MnO2����b�� CuSO4 c��Na2SO3 d��FeCl3
(5)W��������ڵ�ͬ����Ԫ�ء����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
| ��� | ���� | ��ѧ����ʽ |
| ʾ�� | ������ | H2WO3��3H3PO3===3H3PO4��H2W�� |
| 1 |
|
|
| 2 |
|
|
(6)�ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
X��Һ��Y��Һ��Ӧ�����ӷ���ʽ______________��
N���ĵ��ʵĻ�ѧ����ʽΪ____________��
M��Һ������Ũ���ɴ�С������˳����______________ ��
M�������ӵļ������� __________��
(18��)�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
(1)�ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ����________________��
(2)�ڡ��ߵ���ۺ��������������ǿ�����ģ���ԭ�ӽṹ����ԭ��
__________��ԭ�Ӱ뾶�����õ��������������ǽ�����������
(3)�١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д������һ�ֻ�����ĵ���ʽ_______________��
(4)�ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬�������ʲ������÷�Ӧ��������(�����)________��
a��MnO2����b�� CuSO4 c��Na2SO3 d��FeCl3
(5) W��������ڵ�ͬ����Ԫ�ء����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
|
��� |
���� |
��ѧ����ʽ |
|
ʾ�� |
������ |
H2WO3��3H3PO3===3H3PO4��H2W�� |
|
1 |
|
|
|
2 |
|
|
(6)�ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��

X��Һ��Y��Һ��Ӧ�����ӷ���ʽ______________��
N���ĵ��ʵĻ�ѧ����ʽΪ____________��
M��Һ������Ũ���ɴ�С������˳����______________ ��
M�������ӵļ������� __________��
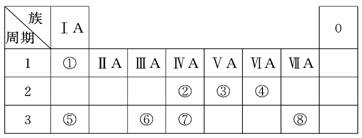
������18�֣��±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�:
|
���� ���� |
��A |
��A |
��A |
��A |
��A |
��A |
��A |
0�� |
|
2 |
|
|
|
�� |
�� |
�� |
|
|
|
3 |
�� |
|
�� |
|
|
�� |
�� |
�� |
|
4 |
�� |
|
|
|
|
|
|
|
(1) ����ЩԪ����,��ѧ��������õ���: (�����Ԫ�ط���)��ԭ�ӽṹʾ��ͼΪ_____________________ ��
(2) ������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��_______��������ǿ�Ļ�����ĵ���ʽ��:_____________��
(3) ��������������������Ԫ����_________��д���������������������Ʒ�Ӧ�����ӷ���ʽ_____________________________________________��
(4) �õ���ʽ��ʾԪ�آ���Ļ�������γɹ��̣� ���û��������� (�� �����ۡ������ӡ�)�����
��5����ʾ����ߵĻ�����ĵ���ʽ ���û��������� ������ԡ��Ǽ��ԡ������γɵġ�
������18�֣��±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�:
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 |
|
|
| �� | �� | �� |
|
|
| 3 | �� |
| �� |
|
| �� | �� | �� |
| 4 | �� |
|
|
|
|
|
|
|
(1) ����ЩԪ����,��ѧ��������õ���: (�����Ԫ�ط���)��ԭ�ӽṹʾ��ͼΪ_____________________ ��
(2) ������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��_______��������ǿ�Ļ�����ĵ���ʽ��:_____________��
(3) ��������������������Ԫ����_________��д���������������������Ʒ�Ӧ�����ӷ���ʽ_____________________________________________��
(4) �õ���ʽ��ʾԪ�آ���Ļ�������γɹ��̣� ���û��������� (�� �����ۡ������ӡ�)�����
��5����ʾ����ߵĻ�����ĵ���ʽ ���û��������� ������ԡ��Ǽ��ԡ������γɵġ�
������18�֣��±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�:
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | | | �� | �� | �� |
| 4 | �� | | | | | | | |
(2) ������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��_______��������ǿ�Ļ�����ĵ���ʽ��:_____________��
(3) ��������������������Ԫ����_________��д���������������������Ʒ�Ӧ�����ӷ���ʽ_____________________________________________��
(4) �õ���ʽ��ʾԪ�آ���Ļ�������γɹ��̣� ���û��������� (�� �����ۡ������ӡ�)�����
��5����ʾ����ߵĻ�����ĵ���ʽ ���û��������� ������ԡ��Ǽ��ԡ������γɵġ�