��Ŀ����
��֪����������ˮ�е��ܽ��Ϊ��0.18g��4�棩��0.34g��25�棩��6.8g��95�棩�����ѵķе�Ϊ34.6�档ʵ���ҳ��ñ���ȩ�Ʊ����״��ͱ����ᣬ��ԭ��Ϊ��2C6H5�DCHO��NaOH C6H5�DCH2OH��C6H5�DCOONa
C6H5�DCH2OH��C6H5�DCOONa
ʵ�鲽�����£�
������ͼ��ʾװ���м������� NaOH��ˮ�ͱ���ȩ�����ȡ����ȣ�ʹ��Ӧ��ֽ��С�
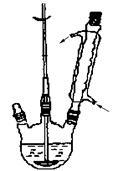
�ڴ��������¿ڼ�����ˮ�����ȣ���ȴ�������Һ©������������ȡ����Һ��ˮ�㱣�����á������Ѳ�������10%̼������Һ��ˮϴ�ӡ�
�۽����Ѳ㵹��ʢ��������ˮ����þ�ĸ�����ƿ�У����ȡ����ú���ת������װ�ã��������ȼ��ȳ�ȥ���ѣ��ռ�198�桫204����ֵñ��״���
�ܽ�������е�ˮ�������Ũ�����Ͼ��ȣ�������ɫ���塣��ȴ�����˵ôֲ�Ʒ�����ֲ�Ʒ�ᴿ�ñ����ᡣ
��1��������У������ˮϴ�ӵ������� ������Һ©��������Һ����뿪��ʵ����������ǣ��� �� ��
��2�����������ˮ����þ�������� ��
��3���������ˮ���Ũ�����Ϻ�����Ӧ�Ļ�ѧ����ʽΪ ������Ӧ��������ȴ��Ŀ���� ��
��4������װ������������������ѹϵͳ�⣬���� �� �����������ƣ���
 C6H5�DCH2OH��C6H5�DCOONa
C6H5�DCH2OH��C6H5�DCOONaʵ�鲽�����£�
������ͼ��ʾװ���м������� NaOH��ˮ�ͱ���ȩ�����ȡ����ȣ�ʹ��Ӧ��ֽ��С�

�ڴ��������¿ڼ�����ˮ�����ȣ���ȴ�������Һ©������������ȡ����Һ��ˮ�㱣�����á������Ѳ�������10%̼������Һ��ˮϴ�ӡ�
�۽����Ѳ㵹��ʢ��������ˮ����þ�ĸ�����ƿ�У����ȡ����ú���ת������װ�ã��������ȼ��ȳ�ȥ���ѣ��ռ�198�桫204����ֵñ��״���
�ܽ�������е�ˮ�������Ũ�����Ͼ��ȣ�������ɫ���塣��ȴ�����˵ôֲ�Ʒ�����ֲ�Ʒ�ᴿ�ñ����ᡣ
��1��������У������ˮϴ�ӵ������� ������Һ©��������Һ����뿪��ʵ����������ǣ��� �� ��
��2�����������ˮ����þ�������� ��
��3���������ˮ���Ũ�����Ϻ�����Ӧ�Ļ�ѧ����ʽΪ ������Ӧ��������ȴ��Ŀ���� ��
��4������װ������������������ѹϵͳ�⣬���� �� �����������ƣ���
B����1����ȥNaOH���������ơ�̼���Ƶ����� ��2�֣�
���²�Һ����¶˷ų������ϲ�Һ����Ͽڵ�����ÿ��1�֣���2�֣�
��2���������2�֣�
��3��C6H5�DCOONa��HCl C6H5�DCOOH��NaCl ��2�֣�
C6H5�DCOOH��NaCl ��2�֣�
ʹ�����ᾡ���ܶ������ ��2�֣�
��4�� ����ƿ ��1�֣� ����©����1�֣�
���²�Һ����¶˷ų������ϲ�Һ����Ͽڵ�����ÿ��1�֣���2�֣�
��2���������2�֣�
��3��C6H5�DCOONa��HCl
 C6H5�DCOOH��NaCl ��2�֣�
C6H5�DCOOH��NaCl ��2�֣�ʹ�����ᾡ���ܶ������ ��2�֣�
��4�� ����ƿ ��1�֣� ����©����1�֣�
��������������ӻ�������ˮ���ܽ�Ƚϴ���ˮ��ȥNaOH���������ơ�̼���Ƶ����ʣ���Һ©����ʹ�ã����²�Һ����¶˷ų������ϲ�Һ����Ͽڵ������𰸣���ȥNaOH���������ơ�̼���Ƶ����� �����²�Һ����¶˷ų������ϲ�Һ����Ͽڵ���������ˮ����þ����ˮ���MgSO4��7H2O������������𰸣�����������������ữ���ã����������Ʊ�ɱ����ᣬ����Һ����������ȴ��Ŀ����ʹ�����ᾡ���ܶ����������߲��ʣ��𰸣�C6H5�DCOONa��HCl
 C6H5�DCOOH��NaCl �� ʹ�����ᾡ���ܶ���������ȳ���װ��������ƿ������©������ɣ��𰸣�����ƿ������©����
C6H5�DCOOH��NaCl �� ʹ�����ᾡ���ܶ���������ȳ���װ��������ƿ������©������ɣ��𰸣�����ƿ������©����
��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д�
�����Ŀ
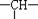 ��һ��-Cl�������ܵĽṹ�� �֡������ⲻ���Ƕ�ӳ�칹�壩
��һ��-Cl�������ܵĽṹ�� �֡������ⲻ���Ƕ�ӳ�칹�壩 ���е���131.4�棬�۵���9.79�棬������ˮ�������ڴ�����ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ�����Ʊ�1��2-�������顣�����Թ�c��װ��Һ�壨���渲������ˮ����
���е���131.4�棬�۵���9.79�棬������ˮ�������ڴ�����ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ�����Ʊ�1��2-�������顣�����Թ�c��װ��Һ�壨���渲������ˮ����