��Ŀ����
��ֽͨ�ŵ���Ҫ�ɷ�����ά�أ������ڵ�ֽ�������У�������ֽ����Ϳ�������Ĺ��գ�����������ף���ֹī����ɢ����ش��������⣺
(1)���Ƿ���ֽ�Żᷢ�����Ը�ʴ����ࡢ����������вֽ������ı��档���������飬�������Ը�ʴ��Ҫ����ֽ��Ϳ�������Ĺ����йأ����еĻ�ѧԭ����
____________________________________________________________________________. (2)Ϊ�˷�ֹֽ�ŵ����Ը�ʴ������ֽ���м���̼��Ƶ����Ӽ����ù���ԭ���Ļ�ѧ����ʽΪ___________________��
Ϊ�˱�����Щֽ��������˽����ȡ���д�ʩ��
(3)����������Һ����ϡ����������Һ��ˮ�ȣ�����������������Ҫ������
___________________________________________________________________________��
(4)����Zn(C2H5)2��Zn(C2H5)2������ˮ��Ӧ��������п�����顣�û�ѧ(����)����ʽ��ʾ�÷�����������п����ֹ���Ը�ʴ��ԭ��________________��
��1������ˮ������Ի����������������£���ά��ˮ�⣬ʹ�߷��������ѡ�
��2��CaCO3��2H�� = Ca2����CO2����H2O
��3�������ļ�ͬ���ᵼ����ά��ˮ�⣬����鼮����
��4��Zn��C2H5��2��H2O = ZnO+2C2H6�� ZnO��2H�� = Zn2+��H2O
��������
�����������1����ά�������������¿���ˮ�⣬������ˮ�������ԡ�
��2��CaCO3�����ᷴӦ
��3����ά���ڼ���������Ҳ��ˮ�⡣
��4��Zn��C2H5��2��ˮ��Ӧ����ZnO��ZnO�����ᷴӦ����ֹ��ĸ�ʴ��
���㣺 ���⿼���˷���ʽ����д����ѧ���յķ�����

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�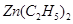 .
.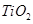 ��
����������Ѱ۵�һ�ֹ�ҵ�Ʒ�������������Ҫ�ɷ�
��
����������Ѱ۵�һ�ֹ�ҵ�Ʒ�������������Ҫ�ɷ�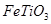 ����Ϊԭ�ϰ��¹��̽��еģ���������л�ѧ����ʽ
����Ϊԭ�ϰ��¹��̽��еģ���������л�ѧ����ʽ