��Ŀ����
��1���Ͼ�����»ᡱ�ѽ��뵹��ʱ���������ݺͳ��н�ͨ�����������ƣ�
���������ݽ���������������ϣ����в��ϲ����ڹ����β��ϵ���______������ĸ����
a��ʯ��ʯ b��ˮ�� c������
�ڹ����ͨ����������������ϣ����н���������������ʴ����______������ĸ����
a�����Ͻ� b������ c����ͭ
��2������»ᡱ�ڼ䣬Ҫ�����˶�Ա��Ӫ���뽡����
�ټ�ʱ�����������˶�Աȡ������ɼ��Ļ�����֤���������Ӫ�����������������������ܵ������ࡢ______��______��
��ˮ�����߲˸���VC����֪VC�ĽṹΪ�������ʽΪ
______�����Ȼ�����Һ�м���VC��Һ����Һ�ɻ�ɫת��Ϊdz��ɫ��˵��VC���н�ǿ��______�ԣ���ѧ��ÿ���貹��Լ60mgά����C�����������к��зḻά����C����______��
a������b������c����d����ù��
�۷���Υ��ҩ�ﲻ���������������Ĺ�ƽ��������Ҳ�к��˶�Ա�����Ľ������ڰ�˾ƥ�֡�
��ù�ء�����ء�С�մ�ȳ���ҩ���У�����ѡ�ֲ��ɷ��õ���______��
��3������ˮ���족����Ϊ�˶�Ա�ṩ����������������չʾ�Ŷ��Ͼ�����������
�����dz���______����ʾ���������������Ǹ��ݿ�����______��______���Ϳ������������Ⱦ���Ũ�ȼ����������ֵ��
��PM2.5ָ�����ڴ����е�ֱ����2.5��m���ף��Ŀ��������PM2.5����ɻ���������Σ�����彡����ȼú���������ڿ���PM2.5�ĺ�����д����̿��ˮ������Ӧ�Ļ�ѧ����ʽ______��
������β���к�����Ⱦ������NO��CO�������������ܼ�װ����ת����������ʹCO��NO��Ӧ����������Ⱦ�����壬��Ӧ�Ļ�ѧ����ʽΪ______��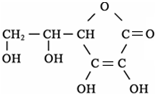
���������ݽ���������������ϣ����в��ϲ����ڹ����β��ϵ���______������ĸ����
a��ʯ��ʯ b��ˮ�� c������
�ڹ����ͨ����������������ϣ����н���������������ʴ����______������ĸ����
a�����Ͻ� b������ c����ͭ
��2������»ᡱ�ڼ䣬Ҫ�����˶�Ա��Ӫ���뽡����
�ټ�ʱ�����������˶�Աȡ������ɼ��Ļ�����֤���������Ӫ�����������������������ܵ������ࡢ______��______��
��ˮ�����߲˸���VC����֪VC�ĽṹΪ�������ʽΪ
______�����Ȼ�����Һ�м���VC��Һ����Һ�ɻ�ɫת��Ϊdz��ɫ��˵��VC���н�ǿ��______�ԣ���ѧ��ÿ���貹��Լ60mgά����C�����������к��зḻά����C����______��
a������b������c����d����ù��
�۷���Υ��ҩ�ﲻ���������������Ĺ�ƽ��������Ҳ�к��˶�Ա�����Ľ������ڰ�˾ƥ�֡�
��ù�ء�����ء�С�մ�ȳ���ҩ���У�����ѡ�ֲ��ɷ��õ���______��
��3������ˮ���족����Ϊ�˶�Ա�ṩ����������������չʾ�Ŷ��Ͼ�����������
�����dz���______����ʾ���������������Ǹ��ݿ�����______��______���Ϳ������������Ⱦ���Ũ�ȼ����������ֵ��
��PM2.5ָ�����ڴ����е�ֱ����2.5��m���ף��Ŀ��������PM2.5����ɻ���������Σ�����彡����ȼú���������ڿ���PM2.5�ĺ�����д����̿��ˮ������Ӧ�Ļ�ѧ����ʽ______��
������β���к�����Ⱦ������NO��CO�������������ܼ�װ����ת����������ʹCO��NO��Ӧ����������Ⱦ�����壬��Ӧ�Ļ�ѧ����ʽΪ______��

��1���ٹ����β��ϰ����������մɡ�ˮ�࣬ʯ��ʯ�����ڹ����β��ϣ��ʴ�Ϊ��a��
�����Ļ�Էdz�ǿ�������¼�����������Ӧ���ڱ����γ����ܵ���������Ĥ����ֹ��һ���������������ڿ����о��н�ǿ�Ŀ���ʴ�ԣ�����һ�γ�ԭ��أ��ӿ츯ʴ��ͭ�����ò��ױ���ʴ���ʴ�Ϊ��b��
��2������������Ӫ���طֱ��ǰ��ʣ����࣬��֬��ά���أ�ˮ�����Σ������������������ܵ������ࡢ��֬�͵����ʣ��ʴ�Ϊ����֬�������ʣ�
�ڸ��ݽ�֪����ʽ��C6H8O6����Һ�ɻ�ɫת��Ϊdz��ɫ˵������������ԭ��ά����C�л�ԭ�ԣ���������ά����C���������������ʣ����������ۣ���ù���ǿ����أ�
�ʴ�Ϊ��C6H8O6����ԭ�ԣ�a��
����ù���ǿ����أ���������˷����ã�С�մ��ǿ���ҩ���ʴ�Ϊ������أ�
��3���������dz��ÿ�����Ⱦָ�� ����ʾ���������������Ǹ��ݿ����ж����������������Ϳ������������Ⱦ���Ũ�ȼ����������ֵ��
�ʴ�Ϊ��������Ⱦָ��������������������
�ڽ�̿��ˮ������Ӧ�Ļ�ѧ����ʽΪC+H2O
CO+H2���ʴ�Ϊ��C+H2O
CO+H2��
��CO��NO��Ӧ�Ļ�ѧ����ʽΪ2CO+2NO
N2+2CO2���ʴ�Ϊ��2CO+2NO
N2+2CO2��
�����Ļ�Էdz�ǿ�������¼�����������Ӧ���ڱ����γ����ܵ���������Ĥ����ֹ��һ���������������ڿ����о��н�ǿ�Ŀ���ʴ�ԣ�����һ�γ�ԭ��أ��ӿ츯ʴ��ͭ�����ò��ױ���ʴ���ʴ�Ϊ��b��
��2������������Ӫ���طֱ��ǰ��ʣ����࣬��֬��ά���أ�ˮ�����Σ������������������ܵ������ࡢ��֬�͵����ʣ��ʴ�Ϊ����֬�������ʣ�
�ڸ��ݽ�֪����ʽ��C6H8O6����Һ�ɻ�ɫת��Ϊdz��ɫ˵������������ԭ��ά����C�л�ԭ�ԣ���������ά����C���������������ʣ����������ۣ���ù���ǿ����أ�
�ʴ�Ϊ��C6H8O6����ԭ�ԣ�a��
����ù���ǿ����أ���������˷����ã�С�մ��ǿ���ҩ���ʴ�Ϊ������أ�
��3���������dz��ÿ�����Ⱦָ�� ����ʾ���������������Ǹ��ݿ����ж����������������Ϳ������������Ⱦ���Ũ�ȼ����������ֵ��
�ʴ�Ϊ��������Ⱦָ��������������������
�ڽ�̿��ˮ������Ӧ�Ļ�ѧ����ʽΪC+H2O
| ||
| ||
��CO��NO��Ӧ�Ļ�ѧ����ʽΪ2CO+2NO
| ||
| ||

��ϰ��ϵ�д�
�����Ŀ
 ��1���Ͼ�����»ᡱ�ѽ��뵹��ʱ���������ݺͳ��н�ͨ�����������ƣ�
��1���Ͼ�����»ᡱ�ѽ��뵹��ʱ���������ݺͳ��н�ͨ�����������ƣ�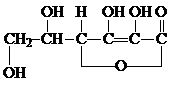 �������ʽΪ
�������ʽΪ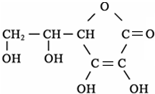 �������ʽΪ
�������ʽΪ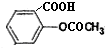 ���dz��õ�
���dz��õ� �������ʽΪ
�������ʽΪ