��Ŀ����
һ���¶��£���3 mol A�����1 mol B����ͨ��һ�ܱ������У��������·�Ӧ��3A(g)+B(g) 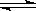 xC(g)������д���пհף�
xC(g)������д���пհף�
��1)����������̶�Ϊ2 L,��Ӧ2minʱ���ʣ��0.6 mol B��C��Ũ��Ϊ0.4 mol/L��
��2min�ڣ�A��ƽ����Ӧ����Ϊ_______________��x= ��
��4min��C��Ũ��___________0.8 mol/L (����ڡ��������ڡ� ��С�ڡ�)��
����֪ƽ��������C���������Ϊ25������B��ת����________��
�ܸı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ���ʼ������������ʵ����ʵ����ֱ�Ϊa��b��c������֮��Ӧ������Ĺ�ϵʽΪ________ ��
��
��2)��ά������ѹǿ���䣺
�ٴﵽƽ��ʱC���������__________25��(����ڡ��������ڡ���С�ڡ�)��
�ڸı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ�����ԭƽ���2������Ӧ����_________mol A�����__________mol B���塣����Ҫ��д������̣�
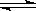 xC(g)������д���пհף�
xC(g)������д���пհף���1)����������̶�Ϊ2 L,��Ӧ2minʱ���ʣ��0.6 mol B��C��Ũ��Ϊ0.4 mol/L��
��2min�ڣ�A��ƽ����Ӧ����Ϊ_______________��x= ��
��4min��C��Ũ��___________0.8 mol/L (����ڡ��������ڡ� ��С�ڡ�)��
����֪ƽ��������C���������Ϊ25������B��ת����________��
�ܸı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ���ʼ������������ʵ����ʵ����ֱ�Ϊa��b��c������֮��Ӧ������Ĺ�ϵʽΪ________ ��
��
��2)��ά������ѹǿ���䣺
�ٴﵽƽ��ʱC���������__________25��(����ڡ��������ڡ���С�ڡ�)��
�ڸı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ�����ԭƽ���2������Ӧ����_________mol A�����__________mol B���塣����Ҫ��д������̣�
��1���� 0.3 ��x= 2 ���� �� �� �� 40% ��
��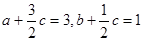
��2���� ���� �� �� 6 �� 2
��
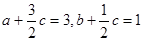
��2���� ���� �� �� 6 �� 2
��1���ٷ�Ӧ2minʱ���ʣ��0.6 mol B��������B��0.4mol��������A��1.2mol������A�ķ�Ӧ������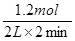 ��0.3mol/(L��min)��ͬʱ����C��0.8mol�����Ը��ݱ仯��֮������Ӧ�Ļ�ѧ������֮�ȿ�֪��x��2��
��0.3mol/(L��min)��ͬʱ����C��0.8mol�����Ը��ݱ仯��֮������Ӧ�Ļ�ѧ������֮�ȿ�֪��x��2��
���������ŷ�Ӧ�Ľ��У���Ӧ���Ũ�����ͣ���˷�Ӧ�������ͣ�����4min��C��Ũ��С��0.8 mol/L��
����ƽ��ʱ����B��bmol��������A��3bmol������C��2bmol����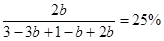 �����b��0.4mol���ת������40����
�����b��0.4mol���ת������40����
�����Ǻ��º��������µĵ�Чƽ�⣬��������ν�����ȣ�ֻҪ��һ�˰������淴Ӧ��ʽ��ȫת��Ϊ��һ�˵����ʺ��൱����ȫ��ͬ����ʼ�����ɡ�����a��b��c֮��Ӧ������Ĺ�ϵʽΪ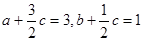 ��
��
��2���������ڷ�Ӧ�����У�����Ǽ�С�ģ�����ѹǿҲ�Ǽ�С����������ڷ�Ӧ�����б��ֺ�ѹ����ﵽƽ��ʱC�������������25����
���������¶Ⱥ�ѹǿ���䣬���������Чƽ�������������֮��A��B�����ʵ���֮�ȵ���3�U1�������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ�����ԭƽ���2������Ӧ����6molA�����2molB���塣
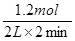 ��0.3mol/(L��min)��ͬʱ����C��0.8mol�����Ը��ݱ仯��֮������Ӧ�Ļ�ѧ������֮�ȿ�֪��x��2��
��0.3mol/(L��min)��ͬʱ����C��0.8mol�����Ը��ݱ仯��֮������Ӧ�Ļ�ѧ������֮�ȿ�֪��x��2�����������ŷ�Ӧ�Ľ��У���Ӧ���Ũ�����ͣ���˷�Ӧ�������ͣ�����4min��C��Ũ��С��0.8 mol/L��
����ƽ��ʱ����B��bmol��������A��3bmol������C��2bmol����
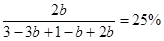 �����b��0.4mol���ת������40����
�����b��0.4mol���ת������40���������Ǻ��º��������µĵ�Чƽ�⣬��������ν�����ȣ�ֻҪ��һ�˰������淴Ӧ��ʽ��ȫת��Ϊ��һ�˵����ʺ��൱����ȫ��ͬ����ʼ�����ɡ�����a��b��c֮��Ӧ������Ĺ�ϵʽΪ
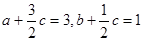 ��
����2���������ڷ�Ӧ�����У�����Ǽ�С�ģ�����ѹǿҲ�Ǽ�С����������ڷ�Ӧ�����б��ֺ�ѹ����ﵽƽ��ʱC�������������25����
���������¶Ⱥ�ѹǿ���䣬���������Чƽ�������������֮��A��B�����ʵ���֮�ȵ���3�U1�������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ�����ԭƽ���2������Ӧ����6molA�����2molB���塣

��ϰ��ϵ�д�
�����Ŀ
 2SO2��g��+ O2 (g)�����й�˵����ȷ����
2SO2��g��+ O2 (g)�����й�˵����ȷ����
 2SO3�����������ܹ�˵����Ӧ�Ѿ��ﵽƽ��״̬���ǣ� ��
2SO3�����������ܹ�˵����Ӧ�Ѿ��ﵽƽ��״̬���ǣ� ��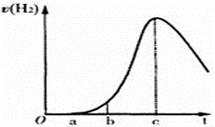
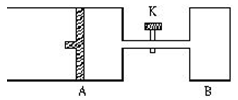
 2Z+2W+Q����H��0��X��Y��Z��W��Ϊ���壩���ﵽƽ��ʱ��VA=1.2aL���Իش�
2Z+2W+Q����H��0��X��Y��Z��W��Ϊ���壩���ﵽƽ��ʱ��VA=1.2aL���Իش� 2C��g���ﵽƽ��ʱ��A��B��C�����ʵ����ֱ�Ϊ4 mol��2 mol��4 mol�������¶Ⱥ��ݻ����䣬��ƽ�����������ߵ����ʵ��������µ�������ʹƽ�����Ƶ��ǣ� ��
2C��g���ﵽƽ��ʱ��A��B��C�����ʵ����ֱ�Ϊ4 mol��2 mol��4 mol�������¶Ⱥ��ݻ����䣬��ƽ�����������ߵ����ʵ��������µ�������ʹƽ�����Ƶ��ǣ� ��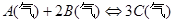 �����������в���˵����Ӧ�Ѵﵽƽ����ǣ� ��
�����������в���˵����Ӧ�Ѵﵽƽ����ǣ� �� 2Z��g���� ��X��Y��Z��ʼŨ�ȷֱ�Ϊc1��c2��c3������Ϊ0��������ƽ��ʱX��Y��Z��Ũ�ȷֱ�Ϊ0.1 mol��L-1��0.3 mol��L-1��0.08 mol��L-1���������жϲ���������(����)
2Z��g���� ��X��Y��Z��ʼŨ�ȷֱ�Ϊc1��c2��c3������Ϊ0��������ƽ��ʱX��Y��Z��Ũ�ȷֱ�Ϊ0.1 mol��L-1��0.3 mol��L-1��0.08 mol��L-1���������жϲ���������(����)