��Ŀ����
��10�֣����ϵ�֣�ݡ�����������������ʹ�����������÷�����з�ʽ�������µ�����ȼ�ϡ��������Ҵ����͡��Ҵ��������ƾ������������ס�С�������Ϊԭ�Ͼ����͡�������Ƴɵġ��Ҵ���һ����ˮ���ټ����������ͺ��γɱ���ȼ���Ҵ����������Ҵ����;��ǰѱ���ȼ���Ҵ������Ͱ�һ�����������γɵij���ȼ�ϡ�����й�֪ʶ������������⣺
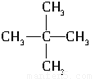
(1)�Ҵ��Ľṹ��ʽΪ_________________����������ʯ�ͷ������õĵͷе�������������е�̼ԭ����һ����C5��C11��Χ�ڣ������飬�����ʽΪ_________________����ͬ���칹��ṹ��ʽ�ֱ�Ϊ_______________________��________________________��_______________________��
(2)�Ҵ����ɺ����ۡ�(C6H10O5)n����ũ��Ʒ�������ס�С������ȣ������͡�������ã��������ơ������Ҫ���������ٵ���+ˮ ������(C6H12O6)
��������
������(C6H12O6)
�������� �Ҵ���
�Ҵ���
��д���������������ǵĻ�ѧ����ʽ��___________________________________��
(3)�Ҵ����ȼ�յIJ���Ϊ_____________��_____________��
(4)�����Ҵ����ͳ�Ϊ����ȼ�ϣ���ԭ����________________________________��
��1��1��5=5�� C2H5OH C5H12 CH3CH2CH2CH2CH3
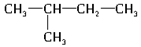
��2��(C6H10O5)n�����ۣ�+nH2O nC6H12O6�������ǣ�
��2�֣�
nC6H12O6�������ǣ�
��2�֣�
��3��CO2 H2O��2�֣�
��4������Ч��������β�����������ش�����Ⱦ�����ƻ��� ��1�֣�
����������1���Ҵ��к����ǻ������Խṹ��ʽΪC2H5OH������������ͨʽCnH2n��2��֪������ķ���ʽ��C5H12������������ͬ���칹�壬�ֱ���CH3CH2CH2CH2CH3��CH3CH2CH(CH3)2��C(CH3)4��
��2������ˮ�⼴���������ǣ�����ʽΪ(C6H10O5)n+nH2O nC6H12O6
nC6H12O6
�����ۣ� �������ǣ�
��3�������Ҵ������Ԫ�ؿ�֪���Ҵ���ȫȼ�յ���������CO2��ˮ��
��4�������Ҵ���ȼ�ղ����֪���Ҵ�����֮���Գ�Ϊ����ȼ�ϣ���ԭ������������Ч��������β�����������ش�����Ⱦ�����ƻ�����

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д� ������(C6H12O6) ��������
������(C6H12O6) ��������