��Ŀ����
����Ŀ�����л���A[CH2=C(CH3)COOCH3]Ϊԭ��,���Է�����ͼ��ʾ��ת����ϵ��

��֪:����������ϡH2SO4�з���ˮ��: CH3COOCH2CH3+H2O![]() CH3COOH+CH3CH2OH
CH3COOH+CH3CH2OH
��ش���������:
(1)B�����к��еĹ�������_____(������)��
(2)��Bת��ΪC�ķ�Ӧ����_____(�����)��
��������Ӧ�����������ڼӳɷ�Ӧ ��ȡ����Ӧ��������������ˮ�ⷴӦ
(3)C��һ�ȴ���D�����ֽṹ,��ṹ��ʽ�ֱ�Ϊ__________��
(4)��A����B�Ļ�ѧ����ʽ��_________________________________________��
���𰸡� �Ȼ���̼̼˫�� �� 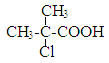
![]()
![]() +H2O
+H2O![]()
![]() +CH3OH
+CH3OH
��������A��ϡ������ˮ�����ɼ״���B����B�Ľṹ��ʽΪCH2=C(CH3)COOH��B���������Ʒ����кͷ�Ӧ����E���ṹ��ʽΪCH2=C(CH3)COONa��B�����������ӳɷ�Ӧ����C���ṹ��ʽΪ(CH3)2CHCOOH��C�������ڹ��������·���ȡ����Ӧ����D����
(1)B�Ľṹ��ʽΪCH2=C(CH3)COOH�������к��еĹ��������Ȼ���̼̼˫����(2)�������Ϸ�����֪��Bת��ΪC�ķ�Ӧ���ڼӳɷ�Ӧ����ѡ�ڡ�(3)C��һ�ȴ���D�����ֽṹ����ṹ��ʽ�ֱ�Ϊ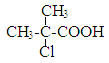 ��
��![]() ��(4)��A����B�Ļ�ѧ����ʽ��
��(4)��A����B�Ļ�ѧ����ʽ��![]() +H2O
+H2O![]()
![]() +CH3OH��
+CH3OH��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���±�ΪԪ�����ڱ���һ���֡���ش��������⣺
�� | |||||||||||||||||
�� | �� | �� | |||||||||||||||
�� | �� | �� | |||||||||||||||
�� | �� | ||||||||||||||||
��1������Ԫ���У�����d������________����Ԫ�ط��ţ���
��2��д��Ԫ�آ�Ļ�̬ԭ�ӵļ۵����Ų�ʽ___________________________��
��3��Ԫ�ص�һ������Ϊ��___�ܣ��縺�Ԣ�___�ܣ��>����<������
��4��Ԫ�آ���̬�⻯�����Ϊ_______���ӣ�����ԡ��Ǽ��ԡ�����������ͭ��Һ����μ�����ˮ��Һ���ɹ۲쵽������Ϊ_______________________________________��
��5��Ԫ�آĵ��ʵľ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��

����֪��ԭ�Ӱ뾶Ϊdcm��NA���������ӵ�������ֵ��Ԫ�آ����ԭ������ΪM����һ�������Т�ԭ�ӵĸ���Ϊ______���þ�����ܶ�Ϊ_________����M��NA��d��ʾ����

