��Ŀ����
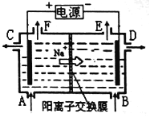
����Ŀ����ͼ����0.1mol/LNaOH��Һ�ֱ�ζ�20mLŨ�Ⱦ�Ϊ0.1mol/L�IJ�ͬһԪ��ĵζ����ߣ�����˵��������ǣ� ��
A�����ԣ�HF>CH3COOH>HCN
B����NaOH��Һ�ζ�����ʱ��Ӧ�÷�̪��ָʾ������ʹ�ü���
C��������10mLNaOH��Һʱ��c(CN-)>c(CH3COO-)
D����NaOH��Һ�ĵ��룬CH3COOH��Һ��ˮ�ĵ���̶��ȱ����С
���𰸡�C
��������
���������A������ͬһŨ�ȵIJ�ͬ�ᣬ����Խǿ������������H+Ũ��Խ����Һ��pHԽС������ͼʾ��֪��Һ��pH:HF<CH3COOH<HCN���������ԣ�HF>CH3COOH>HCN����ȷ��B����NaOH��Һ�ζ�����ʱ������ǡ����ȫ��Ӧ����CH3COONa��ǿ�������Σ�ˮ��ʹ��Һ�Լ��ԣ�����Ӧ�ü��Է�Χ�ڱ�ɫ�ķ�̪��ָʾ����������ʹ�����Է�Χ��ɫ��������ָʾ������ȷ��C��������10mLNaOH��Һʱ����Ӧ�õ�HCN��NaCN��CH3COOH ��CH3COONa��Ũ�ȵĻ����Һ���������ԣ�CH3COOH> HCN ������ˮ��̶ȣ�CN-> CH3COO-��������Һ������Ũ�ȣ�c(CN-)<c(CH3COO-)������D������NaOH��Һ�ĵ��룬CH3COOH��Һ�����Ũ����С�������������ˮ�������������������������ǡ����ȫ��Ӧʱˮ�ĵ���̶ȴﵽ���NaOH��������ˮ�ĵ���ƽ�����������ã�����ˮ�ĵ���̶��ȱ����С����ȷ��

����Ŀ����ҵ����ƽ�VOSO4�е�K2SO4��SiO2���ʳ�ȥ�����յõ�V2O5���������£�

�ش��������⣺
��1����������÷����ijɷ���_____________��д��ѧʽ��������II��������________________��
��2������ڡ��۵ı仯���̱�ʾΪ����ʽR��ʾVO2+��HA��ʾ�л���ȡ������R2(SO4)n(ˮ��)+2nHA(�л���)![]() 2RAn(�л���)+nH2SO4(ˮ��)
2RAn(�л���)+nH2SO4(ˮ��)
����X�Լ�Ϊ_____________��д��ѧʽ����
��3����Ҫ��������з���ʽ
�ܵ����ӷ���ʽΪ_________________________________________��
�Ӱ�ˮ����pH�������漰�����ӷ���ʽΪ�� _________________��_______________��
��4��25ʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
��ʵ�������У����м��백ˮ��������Һ�����pHΪ________������������Ϊ93.1%ʱ������Fe(OH)3����������Һ��c(Fe3+)<_________mol/L����֪��25ʱ��Ksp[Fe(OH)3]=2.610-39����
��5���ù��������У�����ѭ�����õ�������___________��_____________��