��Ŀ����
��һ����ȡ����ͭ
�ٳ�ȡ2gCuSO4?5H2O��ϸ�����ձ�����10mL����ˮ�ܽ⣻
��������CuSO4��Һ����μ���NaOH��Һ��ֱ�����ٲ���������Ȼ�����û����ת�Ƶ���������������ȫ����Ϊ��ɫ��
�۽���������û������ˡ�ϴ�ӣ����ɺ���ϸ���ã�
�ش��������⣺
��1������ʵ�鲽������Ҫʹ�ò���������______����ʵ����ţ�
������֤������ͭ�ܼӿ�����صķֽⲢ��������̵Ĵ�Ч�����бȽ�
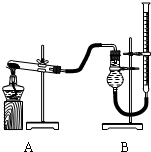
����ͼװ�ý���ʵ�飬ʵ��ʱ��������25mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ�����������£�
| ʵ����� | KClO3���� | ������������ | �������� |
| �� | 1.2g | ���������� | |
| �� | 1.2g | CuO 0.5g | |
| �� | 1.2g | MnO2 0.5g |
��2������ʵ���еġ��������ݡ�ָ______��
��3����Ҫ֤��ʵ����и�������ռ���������O2���ɴ������ռ��������õ��ɼм�סB���齺�ܣ���ȥ������ϵ�����Ƥ����______��
��4��Ϊ̽��CuO��ʵ������Ƿ�������ã��貹������ʵ�飨����д�������������
a��______��b��CuO�Ļ�ѧ������û�иı䣮
�ʴ�Ϊ���٢ڢ�
��2��֤������ͭ�ܼӿ�����صķֽ⣬����������̵Ĵ�Ч�����бȽϣ��Ա�ʵ��һ��Ҫ���Ʊ�������ʵ����Ӧ�����������������������������ͭ��������̵�������ȣ��ɱ���������ɵ������25mL�����������õ�ʱ�䣮
�ʴ�Ϊ������25mL���������ʱ��
��3��������������ȼ�ص㣬���������ķ���Ϊ��ȡһ�������ǵ�ľ�������������ڣ��ɿ����ɼУ���ľ���Ƿ�ȼ��
�ʴ�Ϊ��ȡһ�������ǵ�ľ�������������ڣ��ɿ����ɼУ���ľ���Ƿ�ȼ��
��4���������ܸı仯ѧ��Ӧ�ٶȣ������������ͻ�ѧ���ʲ��䣬�ʻ�Ӧ��������ͭ�������Ƿ�ı䣮
�ʴ�Ϊ��CuO��������û�иı�

 ��У����ϵ�д�
��У����ϵ�д�(һ)��ȡ����ͭ
�ٳ�ȡ
(1)����ʵ�鲽������Ҫʹ�ò���������_______________(��ʵ�����)������٣�������ĥ��������������������_______________��
(2)�������ϴ�ӳ����IJ�����_________________________________________��
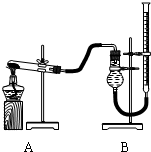
(��)֤������ͭ�ܼӿ�����صķֽⲢ��������̵Ĵ�Ч�����бȽ�����ͼװ�ý���ʵ�飬ʵ��ʱ��������25 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ�������ݼ��±�:
ʵ����� | KClO3���� | ������������ | �������� |
�� | ���������� |
| |
�� | CuO |
| |
�� | MnO2 0.5 |
|

�ش���������:
(3)����ʵ���еġ��������ݡ�ָ_______________��
(4)��Ҫ֤��ʵ����и�������ռ���������O2���ɴ������ռ��������õ��ɼм�סB���齺�ܣ���ȥ������ϵ�����Ƥ����_______________��
(5)Ϊ̽��CuO��ʵ������Ƿ�������ã��貹������ʵ��(����д���������):
a._______________��b.CuO�Ļ�ѧ������û�иı䡣
��14�֣�ijʵ��С������ȡ����ͭ��֤������ͭ�ܼӿ�����صķֽ⣬����������ʵ�飺
��һ����ȡ����ͭ
�ٳ�ȡ2 gCuSO4��5H2O��ϸ�����ձ�����10 mL����ˮ�ܽ⣻
��������CuSO4��Һ����μ���NaOH��Һ��ֱ�����ٲ���������Ȼ�����û����ת�Ƶ���������������ȫ����Ϊ��ɫ��
�۽���������û������ˡ�ϴ�ӣ����ɺ���ϸ���á�
�ش��������⣺
������ʵ�鲽������Ҫʹ�ò���������_______________����ʵ����ţ�������١�������ĥ��������������������___________________��
�Ʋ������ϴ�ӳ����IJ�����______________________________________________
__________________________________________________________��
������֤������ͭ�ܼӿ�����صķֽⲢ��������̵Ĵ�Ч�����бȽ�
����ͼװ�ý���ʵ�飬ʵ��ʱ��������25 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ�������ݼ��±���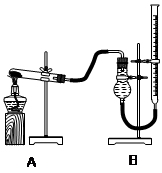
| ʵ����� | KClO3���� | ������������ | �������� |
| �� | 1.2 g | ���������� | |
| �� | 1.2 g | CuO 0.5 g | |
| �� | 1.2 g | MnO2 0.5 g | |
������ʵ���еġ��������ݡ�ָ_____________��
�ȱ�ʵ��װ��ͼ������װ��B�ɸ���ܡ��齺�ܺ�50 mL�ζ��ܸ������װ���ɣ��˴����õζ�����___________�����ʽ����ʽ�����ζ��ܣ�
����Ҫ֤��ʵ����и�������ռ���������O2���ɴ������ռ��������õ��ɼм�סB���齺�ܣ���ȥ������ϵ�����Ƥ����__________________________________________
____________________________________________________��
��Ϊ̽��CuO��ʵ������Ƿ�������ã��貹������ʵ�飨����д�������������
a��_______________________________��b��CuO�Ļ�ѧ������û�иı䡣
ijʵ��С������ȡ����ͭ��֤������ͭ�ܼӿ�����صķֽ⣬����������ʵ�飺
��һ����ȡ����ͭ
�ٳ�ȡ2 gCuSO4��5H2O��ϸ�����ձ�����10 mL����ˮ�ܽ⣻
��������CuSO4��Һ����μ���NaOH��Һ��ֱ�����ٲ���������Ȼ�����û����ת�Ƶ���������������ȫ����Ϊ��ɫ��
�۽���������û������ˡ�ϴ�ӣ����ɺ���ϸ���á�
�ش��������⣺
������ʵ�鲽������Ҫʹ�ò���������_______________����ʵ����ţ�
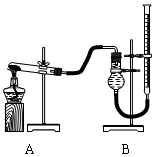
������֤������ͭ�ܼӿ�����صķֽⲢ��������̵Ĵ�Ч�����бȽ�������ͼװ�ý���ʵ�飬ʵ��ʱ��������25 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ�����������£�
| ʵ����� | KClO3���� | ������������ | �������� |
| �� | 1.2 g | ���������� | |
| �� | 1.2 g | CuO 0.5 g | |
| �� | 1.2 g | MnO2 0.5 g | |
������ʵ���еġ��������ݡ�ָ ��
����Ҫ֤��ʵ����и�������ռ���������O2���ɴ������ռ��������õ��ɼм�סB���齺�ܣ���ȥ������ϵ�����Ƥ���� ��
��Ϊ̽��CuO��ʵ������Ƿ�������ã��貹������ʵ�飨����д�������������
a�� ��
b��CuO�Ļ�ѧ������û�иı䡣
 ��2011?�人ģ�⣩ijʵ��С������ȡ����ͭ��֤������ͭ�ܼӿ�����صķֽ⣬����������ʵ�飺
��2011?�人ģ�⣩ijʵ��С������ȡ����ͭ��֤������ͭ�ܼӿ�����صķֽ⣬����������ʵ�飺