��Ŀ����

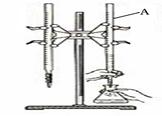
��1������A��������___________��
��2�����������������ζ�ǰ����Ϊ____________mL���ζ������Ϊ_____________mL ��
��3��ijʵ��С��ͬѧ������ʵ���ʵ���������±���ʾ�����ݱ������ݼ�����Ĵ���NaOH��Һ��ƽ��Ũ����_________mol/L��(������λ��Ч����)

A���ü�ʽ�ζ�������ƿ��ע��20��00 mL����NaOH��Һ��������2~3�η�̪��
B���ñ���Һ��ϴ��ʽ�ζ���2~3�Σ�
C����ʢ�б���Һ����ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ��ʹ���������ݣ�
D��ȡ��HCl��Һע����ʽ�ζ������̶�0����2~3 cm��
E������Һ����0��0���¿̶ȣ����¶�����
F������ƿ���ڵζ��ܵ����棬�ñ�HCl��Һ�ζ����յ㲢���µζ���Һ��Ŀ̶ȡ�
��ȷ���������˳����(�������ĸ��д)_________________
��5���ñ�����ζ������ռ���Һ���ζ�ʱ__________����ת��ʽ�ζ��ܵ�___________��_______�ֲ�ͣ����ͬһ����ҡ����ƿ������ע��___________��ֱ��______________Ϊֹ�����жϵζ������յ㡣
��2��0.80��22.80
��3��0.1100
��4��BDCEAF
��5�����ԡ�

 һ����������ϵ�д�
һ����������ϵ�д���ͼ����0.1000 mol/L������ζ�ijδ֪Ũ�ȵ�NaOH��Һ��ʾ��ͼ��ij�εζ�ǰ�����ʢ������ζ�����Һ���λ�á���ش�
�� ����A�������� ��
�� ���������������ζ�ǰ����Ϊ mL���ζ������Ϊ mL ��
�� ijʵ��С��ͬѧ������ʵ���ʵ���������±���ʾ�� ���ݱ������ݼ�����Ĵ���NaOH��Һ��ƽ��Ũ���� mol/L��(������λ��Ч����)


| ʵ�� ��� | ����NaOH��Һ�������mL�� | �ζ�ǰ����� ���������mL�� | �ζ�������� ���������mL�� |
| 1 | 20.00 | 1.20 | 23.22 |
| 2 | 20.00 | 2.21 | 24.21 |
| 3 | 20.00 | 1.50 | 23.48 |
��ͼ����0.1000 mol/L������ζ�ijδ֪Ũ�ȵ�NaOH��Һ��ʾ��ͼ��ij�εζ�ǰ�����ʢ������ζ�����Һ���λ�á���ش�
�� ����A�������� ��
�� ���������������ζ�ǰ����Ϊ mL���ζ������Ϊ mL ��
�� ijʵ��С��ͬѧ������ʵ���ʵ���������±���ʾ�� ���ݱ������ݼ�����Ĵ���NaOH��Һ��ƽ��Ũ���� mol/L��(������λ��Ч����)
| ʵ�� ��� | ����NaOH��Һ�������mL�� | �ζ�ǰ����� ���������mL�� | �ζ�������� ���������mL�� |
| 1 | 20.00 | 1.20 | 23.22 |
| 2 | 20.00 | 2.21 | 24.21 |
| 3 | 20.00 | 1.50 | 23.48 |
�� �ζ������ɷֽ�Ϊ���¼��������õ�������������ˮϴ������
A���ü�ʽ�ζ�������ƿ��ע��20.00 mL����NaOH��Һ��������2~3�η�̪��
B���ñ���Һ��ϴ��ʽ�ζ���2~3�Σ�
C����ʢ�б���Һ����ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ��ʹ���������ݣ�
D��ȡ��HCl��Һע����ʽ�ζ������̶�0����2~3 cm��
E������Һ����0��0���¿̶ȣ����¶�����
F������ƿ���ڵζ��ܵ����棬�ñ�HCl��Һ�ζ����յ㲢���µζ���Һ��Ŀ̶ȡ�
��ȷ���������˳����(�������ĸ��д)________
���ñ�����ζ������ռ���Һ���ζ�ʱ____����ת��ʽ�ζ��ܵ� ��_______�ֲ�ͣ����ͬһ����ҡ����ƿ������ע�� ��ֱ�� Ϊֹ,���жϵζ������յ㡣