��Ŀ����
��һ���¶��£���2molN2��6molH2����̶��ݻ����ܱ������У����� N2(g)��3H2(g) 2NH3(g)���ﵽ��ѧƽ����������¶Ȳ��䣬��a��b��c�ֱ�ΪN2��H2��NH3�����ʵ��������½���ƽ�⣬������и����ʵ����Ժ�����ƽ����ͬ�����
N2(g)��3H2(g) 2NH3(g)���ﵽ��ѧƽ����������¶Ȳ��䣬��a��b��c�ֱ�ΪN2��H2��NH3�����ʵ��������½���ƽ�⣬������и����ʵ����Ժ�����ƽ����ͬ�����
(1)��a=0��b=0����c="������ "
�ơ���a=1�� c =2���� b =��������
�ǡ���a�� b �� cȡֵ���������һ������Ϊ��������ʽ�ӱ�ʾ��һ��ֻ��a��c��һ��ֻ��b��c �������������������� ������������������
(1) 4 (3) 3 (3) 2a+c =" 4 " 2b+3c = 12
����

 С�����ϵ�д�
С�����ϵ�д��������ʵ�����X��Y�������һ�ݻ��ɱ���ܱ������У���һ�������·������·�Ӧ���ﵽƽ�⣺X��g����Y��g����2M��s��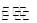 2Z��g����
2Z��g���� �����ı�ij��������ά��������ֱ���ﵽ��ƽ�⣬�±��й�����ƽ����ԭƽ��ıȽϣ���ȷ���� �� ��
�����ı�ij��������ά��������ֱ���ﵽ��ƽ�⣬�±��й�����ƽ����ԭƽ��ıȽϣ���ȷ���� �� ��
|
|
�ı������ |
�����淴Ӧ���ʱ仯 |
��ƽ����ԭƽ��Ƚ� |
|
A |
�����¶� |
�淴Ӧ������������������Ӧ���������� |
X������������� |
|
B |
����ѹǿ |
����Ӧ���������淴Ӧ���ʼ�С |
Z��Ũ�Ȳ��� |
|
C |
��Сѹǿ |
�����淴Ӧ���ʶ���С |
Y������������ |
|
D |
����һ������Z |
�淴Ӧ�������� |
X������������ |
�������ʵ�����X��Y�������һ���ݻ��ɱ���ܱ������У���һ�������·������·�Ӧ���ﵽƽ�⣺X��g����Y��g����2M��s��  2Z��g������H<0�����ı�ij��������ά��������ֱ���ﵽ��ƽ�⣬�±��й�����ƽ����ԭƽ��ıȽϣ���ȷ���� ��
��
2Z��g������H<0�����ı�ij��������ά��������ֱ���ﵽ��ƽ�⣬�±��й�����ƽ����ԭƽ��ıȽϣ���ȷ���� ��
��
|
ѡ�� |
�ı������ |
�����淴Ӧ���ʱ仯 |
��ƽ���ԭƽ��Ƚ� |
|
A |
�����¶� |
�淴Ӧ������������������Ӧ���������� |
X������������ |
|
B |
����ѹǿ |
����Ӧ���������淴Ӧ���ʼ�С |
Z��Ũ�Ȳ��� |
|
C |
��Сѹǿ |
�����淴Ӧ���ʶ���С |
Y������������ |
|
D |
����һ����Z |
�淴Ӧ�������� |
X������������ |
�������ʵ�����X��Y�������һ�ݻ��ɱ���ܱ������У���һ�������·������·�Ӧ���ﵽƽ�⣺X��g����Y��g����2M��s��![]() 2Z��g����
2Z��g����![]() �����ı�ij��������ά��������ֱ���ﵽ��ƽ�⣬�±��й�����ƽ����ԭƽ��ıȽϣ���ȷ���� �� ��
�����ı�ij��������ά��������ֱ���ﵽ��ƽ�⣬�±��й�����ƽ����ԭƽ��ıȽϣ���ȷ���� �� ��
| �ı������ | �����淴Ӧ���ʱ仯 | ��ƽ����ԭƽ��Ƚ� | |
| A | �����¶� | �淴Ӧ������������������Ӧ���������� | X������������� |
| B | ����ѹǿ | ����Ӧ���������淴Ӧ���ʼ�С | Z��Ũ�Ȳ��� |
| C | ��Сѹǿ | �����淴Ӧ���ʶ���С | Y������������ |
| D | ����һ������Z | �淴Ӧ�������� | X������������ |
�������ʵ�����X��Y�������һ���ݻ��ɱ���ܱ������У���һ�������·������·�Ӧ���ﵽƽ�⣺X��g����Y��g����2M��s�� 2Z��g������H<0�����ı�ij��������ά��������ֱ���ﵽ��ƽ�⣬�±��й�����ƽ����ԭƽ��ıȽϣ���ȷ���� �� ��
| ѡ�� | �ı������ | �����淴Ӧ���ʱ仯 | ��ƽ���ԭƽ��Ƚ� |
| A | �����¶� | �淴Ӧ������������������Ӧ���������� | X������������ |
| B | ����ѹǿ | ����Ӧ���������淴Ӧ���ʼ�С | Z��Ũ�Ȳ��� |
| C | ��Сѹǿ | �����淴Ӧ���ʶ���С | Y������������ |
| D | ����һ����Z | �淴Ӧ�������� | X������������ |
�������ʵ�����X��Y�������һ�ݻ��ɱ���ܱ������У���һ�������·������·�Ӧ���ﵽƽ�⣺X��g����Y��g����2M��s�� 2Z��g����
2Z��g���� �����ı�ij��������ά��������ֱ���ﵽ��ƽ�⣬�±��й�����ƽ����ԭƽ��ıȽϣ���ȷ���� �� ��
�����ı�ij��������ά��������ֱ���ﵽ��ƽ�⣬�±��й�����ƽ����ԭƽ��ıȽϣ���ȷ���� �� ��
| | �ı������ | �����淴Ӧ���ʱ仯 | ��ƽ����ԭƽ��Ƚ� |
| A | �����¶� | �淴Ӧ������������������Ӧ���������� | X������������� |
| B | ����ѹǿ | ����Ӧ���������淴Ӧ���ʼ�С | Z��Ũ�Ȳ��� |
| C | ��Сѹǿ | �����淴Ӧ���ʶ���С | Y������������ |
| D | ����һ������Z | �淴Ӧ�������� | X������������ |