��Ŀ����
��ѧ��Ӧ�м������ʱ仯�����������仯���ͷŻ����������ǻ�ѧ��Ӧ�������仯����Ҫ��ʽ֮һ����֪C(ʯī)��H2(g)ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
�� C(ʯī)+ O2(g)��CO(g)
O2(g)��CO(g)  =-111.0 KJ��mol-1
=-111.0 KJ��mol-1
�� H2(g)+
 O2(g) ��H20(g)
O2(g) ��H20(g)  =-242.0 kJ��mol-1
=-242.0 kJ��mol-1
�� C(ʯī)+O2(g)��CO2(g)
 =-394.0 kJ��mol-1
=-394.0 kJ��mol-1
�����������⣺
��1����ѧ��Ӧ���������仯�ı���ԭ���Ƿ�Ӧ�������� �Ķ��Ѻ��γɡ�����������Ӧ���� (����ȡ����ȡ�)��Ӧ��
��2�����Ȼ�ѧ����ʽ�У���Ҫ������Ӧ�P�������״̬��ԭ���� ���ڢ��У�02�Ļ�ѧ��������1/2���DZ�ʾ (����ĸ)��
a�����Ӹ��� b�����ʵ��� c����������
��3����Ӧ2H20(g)��2H2(g)+02(g)�� = KJ��mol-1��
= KJ��mol-1��
��4����C(���ʯ)+02(g)��C02(g)�� =-395.0 kJ��mol-1�����ȶ��ԣ����ʯ (�����������������)ʯī��
=-395.0 kJ��mol-1�����ȶ��ԣ����ʯ (�����������������)ʯī��
��5����֪�γ�H20(g)�е�2 mol H-O���ܷų�926.0 kJ���������γ�1 mol 02(g)�еĹ��ۼ��ܷų�498.0 kJ�������������1 mol H2(g)�е�H-H����Ҫ������ KJ��
��6����ҵ��������һ����Ҫ;������CO(g)��H2O(g)��Ӧ����C02(g)��H2(g)����÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��1����ѧ�� ����
��2����Ӧ���뷴Ӧ�P�������״̬�������йأ������𰸾��ɣ� b
��3��484.0 ��4���� ��5��435.0
��6��CO(g)��H2O(g)��CO2(g)��H2(g) ��H����41 kJ��mol��1
��������
�����������2������״̬��ͬ�����е�������ͬ��״̬֮���ת�������������ı仯�������Ȼ�ѧ����ʽ�б���������ʵ�״̬����3���÷�Ӧ��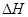 �Ƿ�Ӧ�ڵ�2�����෴�����ɷ���ʽ�жϣ����Ȼ�ѧ����ʽ�еļ�������ָ���ʵ�����û���۵ĺ��壻��4�����ʺ��е�����Խ�࣬�ȶ���Խ����ʯ����ʯī�ų��������ó����ʯ���������ߣ��ȶ��Բ
�Ƿ�Ӧ�ڵ�2�����෴�����ɷ���ʽ�жϣ����Ȼ�ѧ����ʽ�еļ�������ָ���ʵ�����û���۵ĺ��壻��4�����ʺ��е�����Խ�࣬�ȶ���Խ����ʯ����ʯī�ų��������ó����ʯ���������ߣ��ȶ��Բ
��5������1 mol H2(g)�е�H-H����Ҫ������=926.0-498.0��2- 242.0 =435.0 KJ��
��6����Ӧ�ۣ��٣��ڵõ�CO(g)��H2O(g)��CO2(g)��H2(g) ��H����41 kJ��mol��1
���㣺���鷴Ӧ���й����⡣

| A�����Ϸ�Ӧһ���Ƿ��ȷ�Ӧ | B����ѧ��Ӧ�м������ʱ仯���������仯 | C����Ӧ������������������������һ����� | D����ѧ���Ķ��Ѻ��γ��뷴Ӧ���Ⱥ������� |
����˵���У�����ȷ����
| A����ѧ��Ӧ�м������ʱ仯���������仯 |
| B����ʹû�з�����ѧ�仯��Ҳ�����������ı仯 |
| C���κλ�ѧ��Ӧ�е������仯������Ϊ�����仯 |
| D�����ʵĻ�ѧ�ܿ���ͨ����ͬ�ı仯��ʽת��Ϊ���ܡ����ܵ� |