��Ŀ����
��1mol H2(g) ��2mol I2(g) ����2L�ܱ������У���һ���¶��·�����Ӧ��
 H2(g)+ I2(g)
H2(g)+ I2(g) ![]() 2HI(g) ����H��0,���ﵽƽ��,HI�����
2HI(g) ����H��0,���ﵽƽ��,HI�����
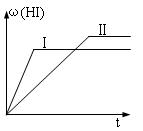
������ʱ��仯��ͼ��II����ʾ�����ı䷴Ӧ��������(HI)
�ı仯������ͼ������ʾ����ı������������ �� ��
A�����º��������£������ʵ�����
B�����º�ѹ�����£�����1 mol N2
C�����������£������¶�
D�����º��������£��ٳ���1 mol H2(g) ��2 mol I2(g)
C
����:
�ı�������(HI)��С��˵��ƽ���������ƶ���A��B��ƶ���D��ԭ״̬���ڵ�Чƽ��״̬���ٷֺ�����ͬ��

��ϰ��ϵ�д�
�����Ŀ

 2HI(g) ����H��0,���ﵽƽ��,HI�����������(HI)��ʱ��仯��ͼ��II����ʾ��
2HI(g) ����H��0,���ﵽƽ��,HI�����������(HI)��ʱ��仯��ͼ��II����ʾ��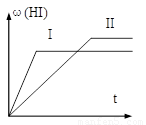
 2HI(g)
��H��0�����ﵽƽ�⣬HI���������
2HI(g)
��H��0�����ﵽƽ�⣬HI��������� (HI)��ʱ��(t)�仯��ͼ����ʾ�����ı䷴Ӧ������
(HI)��ʱ��(t)�仯��ͼ����ʾ�����ı䷴Ӧ������