��Ŀ����
(19��)
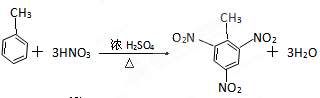
������CH4��C2H4��C2H2��C2H6��C3H8�����л���ش��������⣺
��������ͬʱ:����ͬ״�������������__ ____����ȫȼ��ʱ���ģ�2����������__ __������CO2������___ _������ˮ������___ ___��
��ͬ��ͬѹͬ���ʱ������������ȫȼ������O2����������__
��:�л���ѧ�еķ�Ӧ���ͽ϶࣬�����з�Ӧ���ࣨ����ţ���
���ұ����Ʊ���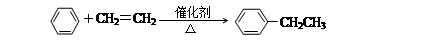
�������ڿ�����ȼ�բ���ϩʹ������Ȼ�̼��Һ��ɫ����ϩʹ���Ը��������Һ��ɫ������ϩ�ƾ���ϩ�����������ڹ��յ������·�Ӧ����������������Ƶ��Ҵ���Һ���Ȣ��ɼױ���ȡTNT
��1����������ȡ����Ӧ���� ��
����������Ӧ���� �����ڼӳɷ�Ӧ���� ��
����ȥ��Ӧ���� ���ھۺϷ�Ӧ����
��2��д���ߺ͢�Ļ�ѧ����ʽ
��
��
��ҽҩ��˹ƥ�ֵĽṹ��ʽ��ͼ1��ʾ��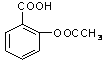

ͼ1 ͼ2
�Ը��ݰ�˹ƥ�ֵĽṹ�ش�
�Ű�˹ƥ�ֿ����������ʣ��ڷ�����θ��ø�������£���˹ƥ�ַ���ˮ�ⷴӦ������A��B �������ʡ�����A�Ľṹ��ʽ��ͼ2��ʾ����B�Ľṹ��ʽΪ ��B�к��еĹ����������� ��
�ư�˹ƥ�ָ�NaHCO3ͬʱ���ã���ʹ����ˮ�����A��NaHCO3��Ӧ�����ɿ�����������Һ�ų����˿������εĽṹ��ʽ��
��д����˹ƥ������������������Һ���ȵĻ�ѧ��Ӧ����ʽ
��:(1)�ã�4���ã�4����2��2�����ã�4���ƣ�3��8
��:�ޢࣻ�ڢܣ��٢ۣ��ߣ��ݣ��������÷֣���ȫ�ߵ�һ��֣�
��2���ߣ�
��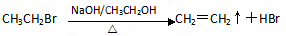
�ࣺ
��1��CH3COOH���Ȼ� ��2��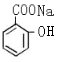
�� +3NaOH ��
+3NaOH �� + CH3COONa +2H2O
+ CH3COONa +2H2O
����

 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д�����A��B��C��D���ֶ����ڷǽ���Ԫ�أ����ǵ�ԭ������������֮��Ϊ19�������ǵĻ������У���Ҫ���ϼ۾���ֹһ�֣�������һ����ͬ�Ļ��ϼۣ�C�ĵ��ʳ�����̫���ܵ�ء����ǵ�һЩ���������£�
| Ԫ�� | A | B | C | D |
| ԭ�Ӱ뾶/nm | 0.102 | 0.077 | 0.117 | 0.075 |
| ���ʵķе�/�� | 444.6 | 4827 | 2355 | �C195.8 |
��1��A��Ԫ�ط���Ϊ__________��DԪ�������ڱ���_______���ڡ���_______�塣
��2���ڳ����£�B��C�����������ֱ�Ϊ��̬��̬��ԭ����____________________��
��3����D�����ֳ�������������Ϸ�Ӧ������һ�����ӻ���������ӻ�����ײ��ʱ�ɷֽ�Ϊ���ַǽ������ʺ�һ�ֻ��������һ��ΪD���ʣ�д���÷ֽⷴӦ�Ļ�ѧ����ʽ____________________________________________________________________��
��4��A��B��C��D���ֱܷ��γɺ�18�����ӵ��⻯���Щ�⻯��ķ���ʽ�ֱ���________________________________________________��
��5��B��D����Ԫ���е����ֻ����ֿ��γɶ��ֺ�14�����ӵĻ�������пռ乹��Ϊֱ���͵���_______________________��_______________________(д�ṹʽ)��
(16��) ����A��B��C��D���ֶ����ڷǽ���Ԫ�أ����ǵ�ԭ������������֮��Ϊ19�������ǵĻ������У���Ҫ���ϼ۾���ֹһ�֣�������һ����ͬ�Ļ��ϼۣ�C�ĵ��ʳ�����̫���ܵ�ء����ǵ�һЩ���������£�
| Ԫ�� | A | B | C | D |
| ԭ�Ӱ뾶/nm | 0.102 | 0.077 | 0.117 | 0.075 |
| ���ʵķе�/�� | 444.6 | 4827 | 2355 | �C195.8 |
��1��A��Ԫ�ط���Ϊ__________��DԪ�������ڱ���_______���ڡ���_______�塣
��2���ڳ����£�B��C�����������ֱ�Ϊ��̬��̬��ԭ����____________________��
��3����D�����ֳ�������������Ϸ�Ӧ������һ�����ӻ���������ӻ�����ײ��ʱ�ɷֽ�Ϊ���ַǽ������ʺ�һ�ֻ��������һ��ΪD���ʣ�д���÷ֽⷴӦ�Ļ�ѧ����ʽ____________________________________________________________________��
��4��A��B��C��D���ֱܷ��γɺ�18�����ӵ��⻯���Щ�⻯��ķ���ʽ�ֱ���________________________________________________��
��5��B��D����Ԫ���е����ֻ����ֿ��γɶ��ֺ�14�����ӵĻ�������пռ乹��Ϊֱ���͵���_______________________��_______________________(д�ṹʽ)��
(16��) ����A��B��C��D���ֶ����ڷǽ���Ԫ�أ����ǵ�ԭ������������֮��Ϊ19�������ǵĻ������У���Ҫ���ϼ۾���ֹһ�֣�������һ����ͬ�Ļ��ϼۣ�C�ĵ��ʳ�����̫���ܵ�ء����ǵ�һЩ���������£�
|
Ԫ�� |
A |
B |
C |
D |
|
ԭ�Ӱ뾶/nm |
0.102 |
0.077 |
0.117 |
0.075 |
|
���ʵķе�/�� |
444.6 |
4827 |
2355 |
�C195.8 |
��1��A��Ԫ�ط���Ϊ__________��DԪ�������ڱ���_______���ڡ���_______�塣
��2���ڳ����£�B��C�����������ֱ�Ϊ��̬��̬��ԭ����____________________��
��3����D�����ֳ�������������Ϸ�Ӧ������һ�����ӻ���������ӻ�����ײ��ʱ�ɷֽ�Ϊ���ַǽ������ʺ�һ�ֻ��������һ��ΪD���ʣ�д���÷ֽⷴӦ�Ļ�ѧ����ʽ____________________________________________________________________��
��4��A��B��C��D���ֱܷ��γɺ�18�����ӵ��⻯���Щ�⻯��ķ���ʽ�ֱ���________________________________________________��
��5��B��D����Ԫ���е����ֻ����ֿ��γɶ��ֺ�14�����ӵĻ�������пռ乹��Ϊֱ���͵���_______________________��_______________________(д�ṹʽ)��
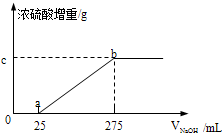 ��2013?������һģ���������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
��2013?������һģ���������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ����� ����PRM������2���źŷ壬��ǿ��֮��Ϊ3��1��
����PRM������2���źŷ壬��ǿ��֮��Ϊ3��1��