��Ŀ����
ұ������һ�������з������ٽ�̿������ˮú��(����������һ����̼)�����ۻ��ý����û������ܵ�ⷨ�����ַ����ڹ�ҵ�Ͼ������á�
��1����ȸʯ��Cu2(OH)2CO3�ݣ���±ʯ(KCl��MgCl2��6H2O)������±��еĽ���ұ���������з��������������ַ�����ѡ����Ӧ�����������пո�
���տ�ȸʯ��ͭ��������Ӧ�Ļ�ѧ����ʽ��д��Ҫ����������
��
��
��2�����پٳ�ʹ����������ұ�����������Ӹ�һ�����û�ѧ����ʽ��ʾ����
�� ��
�� ��
�� ��
��1����ȸʯ��Cu2(OH)2CO3�ݣ���±ʯ(KCl��MgCl2��6H2O)������±��еĽ���ұ���������з��������������ַ�����ѡ����Ӧ�����������пո�
| ���տ�ȸʯ��ͭ | ʪ����ͭ | ���ȷ����� | �ӹ�±ʯ����þ |
| | | | |
��
��
��2�����پٳ�ʹ����������ұ�����������Ӹ�һ�����û�ѧ����ʽ��ʾ����
�� ��
�� ��
�� ��
��1���� �� �� ��
Cu2(OH)2CO3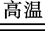 2CuO��H2O��CO2��
2CuO��H2O��CO2��
C��2CuO 2Cu��CO2��
2Cu��CO2��
��2����Fe2O3��3CO 2Fe��3CO2��
2Fe��3CO2��
WO3��3H2 W��3H2O
W��3H2O
��3MnO2��4Al 3Mn��2Al2O3
3Mn��2Al2O3
��2NaCl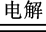 2Na��Cl2��
2Na��Cl2��
Cu2(OH)2CO3
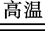 2CuO��H2O��CO2��
2CuO��H2O��CO2��C��2CuO
 2Cu��CO2��
2Cu��CO2����2����Fe2O3��3CO
 2Fe��3CO2��
2Fe��3CO2��WO3��3H2
 W��3H2O
W��3H2O��3MnO2��4Al
 3Mn��2Al2O3
3Mn��2Al2O3��2NaCl
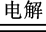 2Na��Cl2��
2Na��Cl2����̿��������һ����̼�dz�ʹ�õĻ�ԭ���������١���һ��ұ�������û�ϻ��õĽ�������ͭ�����ȡ����ý����û�����һ����ˮ��Һ�н��У�Ҳ�����Ƿ�ĩ״���ڹ�ҵ���������ȷ�Ӧ����������ԭ��ұ�����ܵĽ������緰�������̵ȡ����õĽ���һ����õ��ķ�����������ڵ��Ȼ��ơ��Ȼ�þ����������ȡ�ơ�þ������
����ȸʯ����Ҫ�ɷ���Cu2(OH)2CO3�����ȷֽ⣺
Cu2(OH)2CO3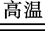 2CuO��H2O��CO2��
2CuO��H2O��CO2��
����ʱ��ʹ�õ���ľ��Ϊľ̿��CuO����ľ̿��Ӧ������ͭ�����ڽ�̿����
C��2CuO 2Cu��CO2�� ���Ϣ�
2Cu��CO2�� ���Ϣ�
����ʪ����ͭ���ҹ�����ʱ��������ijЩ��ͭ���������Һ���û���ͭ�������ý����û�����
Fe��CuSO4 = FeSO4��Cu �ʺ��ڢ�
������ʹ���ۺ�Cr2O3�Ļ�����ڸ����·�Ӧ���û���������Ӧ����������ʹ���ۻ������롣
2Al��Cr2O3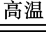 Al2O3��2Cr �ʺ��ڢ�
Al2O3��2Cr �ʺ��ڢ�
�������ӹ�±ʯ��KCl��MgCl2��6H2O���пɵõ���ˮ���Ȼ�þ���壬�ٲ���ͨ��ֽ����ڵ��Ȼ�þ�ķ�����ȡþ��
MgCl2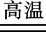 Mg��Cl2�� ���Ϣ�
Mg��Cl2�� ���Ϣ�
����ȸʯ����Ҫ�ɷ���Cu2(OH)2CO3�����ȷֽ⣺
Cu2(OH)2CO3
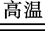 2CuO��H2O��CO2��
2CuO��H2O��CO2������ʱ��ʹ�õ���ľ��Ϊľ̿��CuO����ľ̿��Ӧ������ͭ�����ڽ�̿����
C��2CuO
 2Cu��CO2�� ���Ϣ�
2Cu��CO2�� ���Ϣ�����ʪ����ͭ���ҹ�����ʱ��������ijЩ��ͭ���������Һ���û���ͭ�������ý����û�����
Fe��CuSO4 = FeSO4��Cu �ʺ��ڢ�
������ʹ���ۺ�Cr2O3�Ļ�����ڸ����·�Ӧ���û���������Ӧ����������ʹ���ۻ������롣
2Al��Cr2O3
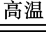 Al2O3��2Cr �ʺ��ڢ�
Al2O3��2Cr �ʺ��ڢ��������ӹ�±ʯ��KCl��MgCl2��6H2O���пɵõ���ˮ���Ȼ�þ���壬�ٲ���ͨ��ֽ����ڵ��Ȼ�þ�ķ�����ȡþ��
MgCl2
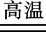 Mg��Cl2�� ���Ϣ�
Mg��Cl2�� ���Ϣ�
��ϰ��ϵ�д�
�����Ŀ
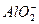 +2H2O�Ĺ��̷�Ӧ����
+2H2O�Ĺ��̷�Ӧ����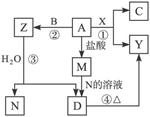
 �������������������ˮ��Ӧ�����ף����ж�������Ե�ǿ��
�������������������ˮ��Ӧ�����ף����ж�������Ե�ǿ��
