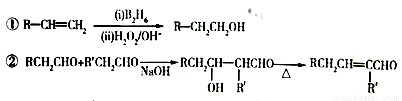
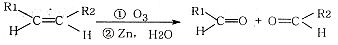��Ŀ����
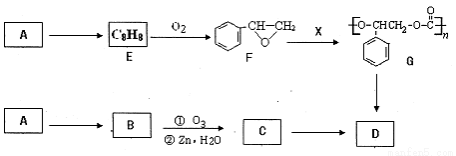
�л���M��һ��ʳƷ���Ͽ���C4Hl0Ϊԭ��ͨ������·�ߺϳɣ�
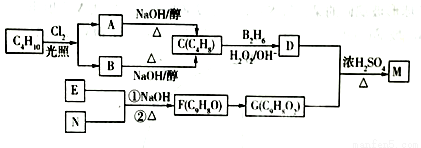
��֪��
��F�����е�̼����û��֧����EΪ�����廯������E��N���ܷ���������Ӧ��
��ش��������⣺
��1��A��B��Ϊһ�ȴ�����д������һ�ֵ����ƣ�ϵͳ��������________��M�еĹ���������Ϊ_______���������⣩��
��2��д�����з�Ӧ�ķ�Ӧ���ͣ�F��G____________��D+G��M______________��
��3��F������Cu(OH)2/NaOH(aq)��Ӧ�Ļ�ѧ����ʽΪ��_________________��
��4��M�Ľṹ��ʽΪ_____________��E�ĺ˴Ź���������______�ֲ��塣
��5����G������ͬ�����ŵ�G�ķ�����ͬ���칹����_______�֣���д������һ�ֵĽṹ��ʽ______��
��6�����������ϳ�·�ߣ��Ա���ϩ��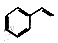 ������ȩΪԭ�ϣ����Լ���ѡ��������Ʊ�
������ȩΪԭ�ϣ����Լ���ѡ��������Ʊ�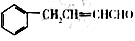 �ĺϳ�·��________________��
�ĺϳ�·��________________��
��ϰ��ϵ�д�
�����Ŀ