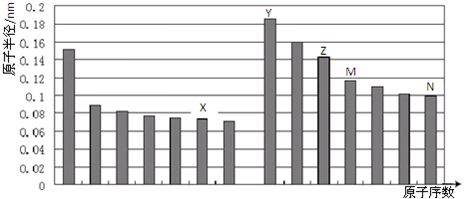
��Ŀ����
ij���ʳ����Ϳ����������������������Ϊ0.2��Ϊԭ����������粒������£�
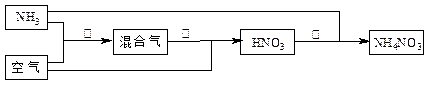
���з�Ӧ��Ϊ4NH3��5O2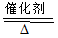 4NO��6H2O
4NO��6H2O
�Ų�����з�����������Ӧ������������ѧ����ʽ�ϲ�Ϊһ����ѧ����ʽ���ɱ�ʾΪ_____________
________________________��
���������Ǹ���Ӧ�Ҹ�����Ӧ����ȫ��Ϊʹ���������в��ٲ����������ԭ�����а��������ڢ۲����������յİ���������������ֵΪ____________��
�Ǽ���ʵ�������У���Ӧ�١����к������ʵ������ʷֱ�Ϊa��b����Ӧ���а���������Ϊc�������������Ϊ100������ϳ�����淋����������У��������������Ƕ��٣�
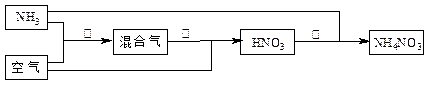
���з�Ӧ��Ϊ4NH3��5O2
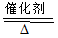 4NO��6H2O
4NO��6H2O�Ų�����з�����������Ӧ������������ѧ����ʽ�ϲ�Ϊһ����ѧ����ʽ���ɱ�ʾΪ_____________
________________________��
���������Ǹ���Ӧ�Ҹ�����Ӧ����ȫ��Ϊʹ���������в��ٲ����������ԭ�����а��������ڢ۲����������յİ���������������ֵΪ____________��
�Ǽ���ʵ�������У���Ӧ�١����к������ʵ������ʷֱ�Ϊa��b����Ӧ���а���������Ϊc�������������Ϊ100������ϳ�����淋����������У��������������Ƕ��٣�
��1��4NO��3O2��2H2O��4HNO3
��2��1/6����16.7%��
��2��1/6����16.7%��
���⿼�����ڹ�ҵ���̱����µĻ�ѧ���㣬�ؼ����ܹ�ȷ���ҳ�������ԭ�ϺͲ����Ĺ�ϵ��Ȼ��������ҵ��ı�����ϵ������Ӧ����.�ҹ�ϵʽ��һ�ַ������ǽ�����ʽ��������ʽ������Ӧ����Ԫ���ó�������Ҫ��������ʵĹ�ϵ��
�ŷ�Ӧ���漰����������Ӧ�� 2NO+O2 = 2NO2 I
3NO2+H2O = 2HNO3+NO II
I��3+II��2����ȥ�м���NO2���� ��4NO+3O2 +2H2O = 4HNO3
���������̰�������������Ӧ��
4NH3��5O2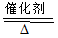 4NO��6H2O ��
4NO��6H2O ��
4NO+3O2 +2H2O = 4HNO3 ��
NH3��HNO3 = NH4NO3 ��
��+ ��+�ۡ�4��Լ��ã� 2NH3��+2O2 = NH4NO3 + H2O
��n(NH3)��n(O2)=1��1 ��֪V(NH3)��V(����)=1��5
��3��
�ⷨһ����������NH3�������ʵ���Ϊ1mol������������ȡHNO3��NH3�����ʵ���Ϊx mol����HNO3���յ�NH3�����ʵ���Ϊymol�����У�x��y��1��abx��cy��
��ã�x��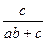 y��
y��
�����������ʣ���xab��yc����1��
�ⷨ��������ڢٲ��μӷ�Ӧ�İ��������ʵ���Ϊ4mol����������������ʵ���4abmol��
�ڢ۲���Ҫ���������ʵ���Ϊ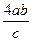 mol�� �����������ʣ�
mol�� �����������ʣ�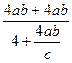 ��
��
�ŷ�Ӧ���漰����������Ӧ�� 2NO+O2 = 2NO2 I
3NO2+H2O = 2HNO3+NO II
I��3+II��2����ȥ�м���NO2���� ��4NO+3O2 +2H2O = 4HNO3
���������̰�������������Ӧ��
4NH3��5O2
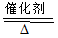 4NO��6H2O ��
4NO��6H2O ��4NO+3O2 +2H2O = 4HNO3 ��
NH3��HNO3 = NH4NO3 ��
��+ ��+�ۡ�4��Լ��ã� 2NH3��+2O2 = NH4NO3 + H2O
��n(NH3)��n(O2)=1��1 ��֪V(NH3)��V(����)=1��5
��3��

�ⷨһ����������NH3�������ʵ���Ϊ1mol������������ȡHNO3��NH3�����ʵ���Ϊx mol����HNO3���յ�NH3�����ʵ���Ϊymol�����У�x��y��1��abx��cy��
��ã�x��
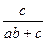 y��
y��
�����������ʣ���xab��yc����1��

�ⷨ��������ڢٲ��μӷ�Ӧ�İ��������ʵ���Ϊ4mol����������������ʵ���4abmol��
�ڢ۲���Ҫ���������ʵ���Ϊ
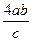 mol�� �����������ʣ�
mol�� �����������ʣ�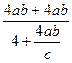 ��
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ