��Ŀ����
����Ŀ����ҵ����CO����ȼ�ϼ״���һ�������·�����Ӧ:CO��g��+2H2��g��![]() CH3OH��g����
CH3OH��g����
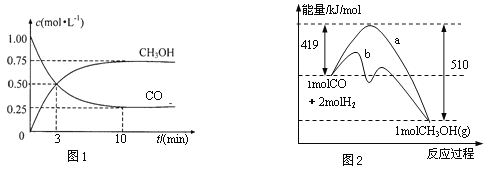
��1��ͼ1�DZ�ʾһ���¶��£������Ϊ2 L���ܱ������м���4 molH2��һ������CO����CO��CH3OH��g����Ũ����ʱ������������ӷ�Ӧ��ʼ��ƽ�⣬��COŨ�ȱ仯��ʾƽ����Ӧ���� v��CO��=_____________��H2��ƽ��ת����Ϊ_______________��
��2��ͼ2��ʾ�÷�Ӧ���й����������ı仯������a��ʾ��ʹ�ô���ʱ��Ӧ�������仯������b��ʾʹ�ô�����������仯��д����Ӧ������ѧ����ʽ:______________��
��3�����¶�������Ӧƽ�ⳣ��K=______________������ֵ����
��4�����������£����д�ʩ����ʹ��Ӧ��ϵ��![]() ����Ĵ�ʩ��____________��
����Ĵ�ʩ��____________��
A�������¶� B������He�� C���ٳ���2molH2 D��ʹ�ô���
���𰸡���1��0.075mol/��Lmin����75%��
��2��CO��g��+H2��g��![]() CH3OH��g�� ��H=-91kJ/mol����3��12����4��C��
CH3OH��g�� ��H=-91kJ/mol����3��12����4��C��
��������
�����������1������ͼ���֪��Ӧ���е�10minʱ�ﵽƽ��״̬����ʱ���ɼ״�0.75mol/L������ݷ���ʽ��֪����CO��Ũ����0.75mol/L��������CO��ʾ��ƽ����Ӧ����Ϊ0.75mol/L��10min��0.075 mol��L��1��min��1������������1.5mol/L����������ʼŨ����2mol/L�����������ת������![]() ��100%��75%��
��100%��75%��
��2������ͼ���֪��Ӧ����������������������������˷�Ӧ�Ƿ��ȷ�Ӧ����Ӧ�ȣ�419kJ/mol��510kJ/mol����91kJ/mol�����Ȼ�ѧ����ʽΪCO��g��+2H2��g��![]() CH3OH��g�� ��H����91 kJ��mol��1��
CH3OH��g�� ��H����91 kJ��mol��1��
��3��ƽ��ʱ����������Ũ����0.75mol/L ��2��1.5mol/L����������ʼŨ����4mol��2L��2mol/L������ƽ��ʱ����Ũ����0.5mol/L�������ͼ���֪ƽ�ⳣ��K��![]() ��
��
��4��A�������¶�ƽ�����淴Ӧ������У����ֵ��С��A����B�������ݻ��������He����ƽ�ⲻ�ƶ�������ֵ���䣬B����C���ٳ���2mol����ƽ��������Ӧ������У���˱�ֵ����C��ȷ��D���������ܸı�ƽ��״̬�����Ա�ֵ���䣬D����ѡC��

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�����Ŀ��CO2��Ŀǰ����Ҫ��������������СCO2���ŷŲ�������������ֵ�Ļ�ѧ��Ʒ��Ŀǰ���о�Ŀ�ꡣ
��1������CO2��CH4�����ϳ���(CO��H2)��
��֪��CH4(g)+2O2(g)![]() CO2(g)+2H2O(g) ��H=��890.3 KJ��mol��1
CO2(g)+2H2O(g) ��H=��890.3 KJ��mol��1
CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H=+2.8 KJ��mol-1
CO2(g)+H2(g) ��H=+2.8 KJ��mol-1
2CO(g)+O2(g)![]() 2CO2(g) ��H=��566.0 KJ��mol��1
2CO2(g) ��H=��566.0 KJ��mol��1
��ӦCO2(g)+CH4(g)![]() 2CO(g)+2H2(g) ��H= ____________��
2CO(g)+2H2(g) ��H= ____________��
��250��ʱ�������Ͻ�Ϊ�����������Ϊ4 L���ܱ�������ͨ��6 mol CO2��6 mol CH4����ʼ�������·�Ӧ��CO2(g)+CH4(g)![]() 2CO(g)+2H2(g)������һ��ʱ��ﵽƽ�⣬ƽ����ϵ�и�����������(ijһ�ɷ����ʵ���ռ���������ʵ����İٷ���)���±���
2CO(g)+2H2(g)������һ��ʱ��ﵽƽ�⣬ƽ����ϵ�и�����������(ijһ�ɷ����ʵ���ռ���������ʵ����İٷ���)���±���
���� | CH4 | CO2 | CO | H2 |
������� | 0.1 | 0.1 | 0.4 | 0.4 |
���¶��¸÷�Ӧ��ƽ�ⳣ��K=________________��
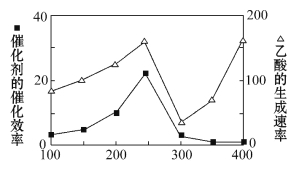
��2���Զ������ѱ��渲��Cu2Al2O4Ϊ���������Խ�CO2��CH4ֱ��ת�����������ڲ�ͬ�¶��´����Ĵ�Ч���������������������ͼ��ʾ��250��300��ʱ��������������ʼ�С������ԭ����____________________��
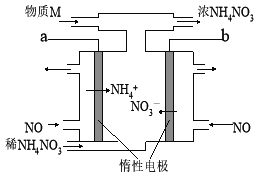
��3�� ������������ˮ��Һ������ʽ��е����CO2��ͭ�缫�Ͽ�ת��Ϊ�������õ缫��Ӧ����ʽΪ_____________________��
��4����2mol CO2��6molH2�ݻ���ͬ���¶Ȳ�ͬ�����������������ܱ������п�ʼ������Ӧ��CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g)�����CH3OH�����ʵ�����ʱ��ı仯����ͼ1��ʾ��
CH3OH(g)+H2O(g)�����CH3OH�����ʵ�����ʱ��ı仯����ͼ1��ʾ��

��������������Ӧ��ƽ�ⳣ����С��ϵΪK��_______K��(�>����=����<��)����֪�� ��Ӧ��һ��_______(��������������������)��Ӧ��
��������ʵ˵���÷�Ӧ�Ѵﵽƽ��״̬����_________________��
A������������ѹǿ���ֲ���
B��������������ܶȱ��ֲ���
C��CO2������������ֲ���
D��CO2������������CH3OH�������������
E�������ڻ�������ƽ����Է����������ֲ���
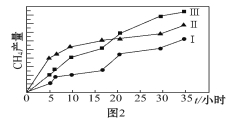
��5�����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ���ڲ�ͬ����(����������)�����£�CH4���������ʱ��ı仯����ͼ2��ʾ����0��15Сʱ�ڣ�CH4��ƽ���������������������Ӵ�С��˳��Ϊ_____________(�����)��