��Ŀ����
(12�֡�ij�о���ѧϰС����̽��SO2�ܷ���BaCl2��Һ��Ӧ����BaSO3�������������ϵ�֪������BaSO3��KSPΪ��������������
��
(1)��0.1 mol• L��1��BaCl2��Һ���뱥���������У�_______(��ܡ����ܡ�������BaSO3������ԭ����______________(��д����Ҫ���ƶϹ��̣���
(2)Ũ����ķе�Ϊ338��C���ƾ��ƻ�����¶�Ϊ400〜5000C����ͬѧ��װ��I����ʵ�飬����BaCl2��Һ�г��ְ�ɫ�������Ұ�ɫ�������������ᡣ
��д�������Թ��з�����Ӧ�Ļ�ѧ����ʽ��_____________________
�ڰ�ɫ�����Ļ�ѧʽ��_______���������ӷ���ʽ��ʾ���ɸð�ɫ�����Ŀ���ԭ��___________________________________
(3)��ͬѧ��Ϊ��ͬѧ��װ�ò����ƣ�����˸Ľ�װ��II����ʵ�飨�г�װ�ú�A�м���װ�����ԣ��������Ѽ��飩��
�ٴ��ɼУ�ͨ��N2����ʱ���رյ��ɼ�
�ڵμ�һ����Ũ���ᣬ����A��һ��ʱ���C��δ���������ɡ�
�����ٵ�Ŀ����_______��ϴ��ƿB�е��Լ���______________��
(4)��ͬѧȡ��ʵ����C����Һ�������μ�һ����ɫ��Һ��Ҳ��������������İ�ɫ���������μӵ��Լ�������______________��
A. NaOH ��Һ B. Na[Al(OH)4]��Һ C. H2O2 ��Һ D.���� KMnO4 ��Һ
��12�֣���1�����ܣ�1�֣� ��ʱ��Һ��c(Ba2+)��0.1 mol�� L-1 �� c(SO32-)��6.3��10-8mol�� L-1��
��Ũ����Q=c(Ba2+)��c(SO32-)��0.1��6.3��10-8=6.3��10-9��Ksp(BaSO3)=5.48��10-7��1�֣�
��2���� Cu + 2H2SO4(Ũ) CuSO4+SO2��+ 2H2O��2�֣�
�� BaSO4��1�֣�
Ba2++ SO42-= BaSO4����2�֣� 2 Ba2++2SO2 + O2+2H2O= 2BaSO4��+ 4H+��2�֣�
��3���ų�װ���ڵĿ�����O2����1�֣� ����NaHSO3��Һ��1�֣� ��4��C��1�֣�
����:���⿼�����ܵ���ʳ����ܽ�ƽ�⡣������Һ��ϡ����Һ��c(Ba2+)��0.1 mol�� L-1 �� c(SO32-)��6.3��10-8mol�� L-1����Ũ����Q=c(Ba2+)��c(SO32-)��0.1��6.3��10-8=6.3��10-9��Ksp(BaSO3)=5.48��10-7���ʲ�������BaSO3������(2) ��Ũ������ͭ��Ӧ�ķ���ʽΪCu+2H2SO4(Ũ) CuSO4+ SO2��+2H2O������SO2��BaCl2��Һ���ò��������������ݾƾ��Ƶĵ��¶ȣ���֪��Ũ����������������BaCl2��Һ��������Ӧ����BaSO4���������ӷ���ʽΪBa2++ SO42-=BaSO4������3��ͨ��N2��Ŀ�����ų�װ���ڵĿ�����O2������ֹO2��SO2��Ӧ����SO3��Ӱ��ʵ���������ñ���NaHSO3��Һ��ȥ������������ֹ��������Ӱ��ʵ������(4) ��ʵ����C����Һ��BaCl2������������Һ���Ӽ�����������ܲ������������Ӽ�����ij�������Һ�����ᣬ������ KMnO4 ��Һ����ɫ����ѡC��

�����12�֣�ij�о���ѧϰС��Ϊ�о�Cu��ŨH2SO4�ķ�Ӧ���������ʵ��̽��������װ���еĹ̶������;ƾ��ƾ�δ������
ʵ��ѡ��ϸͭ˿��98.3% H2SO4��Ʒ����Һ������ʯ��ˮ��CCl4��NaOH��Һ��ҩƷ��ͭ˿����������״��һ��û��ŨH2SO4�У���һ��¶����Һ���Ϸ���
|
�����������ϻش���������
��1��A�Թ��Ϸ��ij����ܵ�������_________________��D��E��֧�Թ���CCl4��������_____________��
��2�����ȹ����У��۲쵽A�Թ��г��ִ�����ɫ��������������������Թ��ϲ��ڱ���������ɫ�������ʣ��ڳ�������Ũ���ᣨ���ڣ�ʱ������ɫ������������������ʧ��д������ɫ������ʧ�Ļ�ѧ��Ӧ����ʽ��____��
��3����A�Թ��е�ŨH2SO4��ͭ˿���м��ȣ��ܿ췢��C�Թ���Ʒ����Һ��ɫ����ʼ��δ��D�Թ��г���ʯ��ˮ���ֻ��ǻ��������IJ����ǣ�___________�����ʵ����֤��IJ���________________��
��4�����������о��������ѧ֪ʶ������ΪҺ���·�ͭ˿����ĺ�ɫ���ʳɷ���_____����д��ѧʽ��
�����12�֣�ij�о���ѧϰС��Ϊ�о�Cu��ŨH2SO4�ķ�Ӧ���������ʵ��̽��������װ���еĹ̶������;ƾ��ƾ�δ������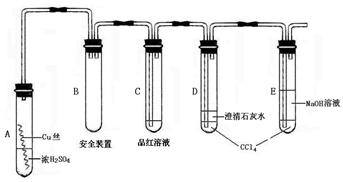
ʵ��ѡ��ϸͭ˿��98.3% H2SO4��Ʒ����Һ������ʯ��ˮ��CCl4��NaOH��Һ��ҩƷ��ͭ˿����������״��һ��û��ŨH2SO4�У���һ��¶����Һ���Ϸ���
|

�����������ϻش���������
��1��A�Թ��Ϸ��ij����ܵ�������_________________��D��E��֧�Թ���CCl4��������_____________��
��2�����ȹ����У��۲쵽A�Թ��г��ִ�����ɫ��������������������Թ��ϲ��ڱ���������ɫ�������ʣ��ڳ�������Ũ���ᣨ���ڣ�ʱ������ɫ������������������ʧ��д������ɫ������ʧ�Ļ�ѧ��Ӧ����ʽ��____��
��3����A�Թ��е�ŨH2SO4��ͭ˿���м��ȣ��ܿ췢��C�Թ���Ʒ����Һ��ɫ����ʼ��δ��D�Թ��г���ʯ��ˮ���ֻ��ǻ��������IJ����ǣ�___________�����ʵ����֤��IJ���________________��
��4�����������о��������ѧ֪ʶ������ΪҺ���·�ͭ˿����ĺ�ɫ���ʳɷ���_____����д��ѧʽ��
�����12�֣�ij�о���ѧϰС��Ϊ�о�Cu��ŨH2SO4�ķ�Ӧ���������ʵ��̽��������װ���еĹ̶������;ƾ��ƾ�δ������
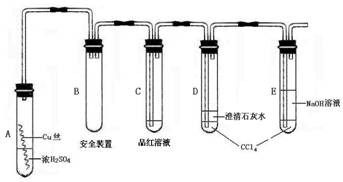
ʵ��ѡ��ϸͭ˿��98.3% H2SO4��Ʒ����Һ������ʯ��ˮ��CCl4��NaOH��Һ��ҩƷ��ͭ˿����������״��һ��û��ŨH2SO4�У���һ��¶����Һ���Ϸ���
|

�����������ϻش���������
��1��A�Թ��Ϸ��ij����ܵ�������_________________��D��E��֧�Թ���CCl4��������_____________��
��2�����ȹ����У��۲쵽A�Թ��г��ִ�����ɫ��������������������Թ��ϲ��ڱ���������ɫ�������ʣ��ڳ�������Ũ���ᣨ���ڣ�ʱ������ɫ������������������ʧ��д������ɫ������ʧ�Ļ�ѧ��Ӧ����ʽ��____��
��3����A�Թ��е�ŨH2SO4��ͭ˿���м��ȣ��ܿ췢��C�Թ���Ʒ����Һ��ɫ����ʼ��δ��D�Թ��г���ʯ��ˮ���ֻ��ǻ��������IJ����ǣ�___________�����ʵ����֤��IJ���________________��
��4�����������о��������ѧ֪ʶ������ΪҺ���·�ͭ˿����ĺ�ɫ���ʳɷ���_____����д��ѧʽ��