��Ŀ����
3��̼�͵��Ƕ�ֲ�����е���Ҫ���Ԫ�أ�������й����ŷŶ�����̼���������ЧӦ���������������⻯ѧ������Ŀǰ����Щ�ж��к�����Ĵ�����Ϊ��ѧ�о�����Ҫ���ݣ���1���û���̿��ԭ��������������йط�ӦΪ��C��s��+2NO��g��?N2��g��+CO2��g����
ij�о�С����һ�������ݻ�Ϊ3L���ݻ�������ܱ������������������������Բ��ƣ��м���NO�������Ļ���̿���ں��£�T1�棩�����·�Ӧ����ò�ͬʱ�䣨t��ʱ�����ʵ����ʵ�����n�������
| n/mol t/min | NO | N2 | CO2 |
| 0 | 2.00 | 0 | 0 |
| 10 | 1.16 | 0.42 | 0.42 |
| 20 | 0.80 | 0.60 | 0.60 |
| 30 | 0.80 | 0.60 | 0.60 |
�ڸ��ݱ������ݣ�����T1��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ0.56��������λС��������ijһʱ�̣���������1.2molC��1.2molNO��0.75molN2��1.08molCO2����ʱv������=v���棩�����������=����
�����и������жϸ÷�Ӧ�ﵽƽ��״̬����AC���������ĸ����
A��v��NO��������=2v��N2�����棩 B��������CO2��N2�������Ϊ1��1
C����������ƽ����Է����������ֲ��� D��������ѹǿ���ֲ���
��һ���¶��£�����NO����ʼŨ��������NO��ƽ��ת���ʲ��䣨����������䡱��С������
��2����ˮ����ͨ�����ȵ�̼���ɲ���ˮú������֪��C��s��+H2O��g��?CO��g��+H2��g����H=+131.3kJ•mol-1��C��s��+CO2��g��?2CO��g����H=+172.5kJ•mol-1����CO��g��+H2O��g��?CO2��g��+H2��g�����ʱ��H=-41.2kJ•mol-1��
��3����3L�ݻ��ɱ���ܱ������з���������Ӧ��H2��g��+CO2��g��?H2O��g��+CO��g����������c��CO���淴Ӧʱ��t�仯�����ߢ���ͼ��ʾ��
������t0ʱ�ı�һ��������ʹ���ߢ������ߢ���ı�������Ǽ��������

������t0ʱ�̽������������ѹ����2L�������������䣩������ͼ�л���c��CO���淴Ӧʱ��t�仯�����ߣ�
���� ��1����0min��10min�ڵ������ʵ����仯Ϊ0.42mol������v=$\frac{��c}{��t}$����v��N2����
�ڸ��ݱ������ݿ�֪��20minʱ����ƽ��״̬���ٸ���K=$\frac{c��{N}_{2}����c��C{O}_{2}��}{{c}^{2}��NO��}$����ƽ�ⳣ��������Ũ����Qc����ƽ�ⳣ������жϷ�Ӧ���з������ж�v��������v���棩��Դ�С��
��A��ƽ��״̬ʱ��ͬ���ʱ�ʾ����������֮�ȵ��ڻ�ѧ������֮�ȣ�
B�����ڵ����������̼��ѧ������֮��Ϊ1��1��������������������CO2��N2�������ʼ��Ϊ1��1��
C����������ܵ����ʵ������䣬�淴Ӧ���л�������������������������ƽ����Է�����������ƽ����Է����������ֲ���˵������ƽ�⣻
D��������ѹǿʼ�ձ��ֲ��䣻
��һ���¶��£�NO����ʼŨ������ЧΪ����ѹǿ����Ӧǰ����������ʵ������䣬ƽ�ⲻ�ƶ���NO��ת���ʲ��䣻
��2����֪����C��s��+H2O��g��?CO��g��+H2��g����H=+131.3kJ•mol-1��
��C��s��+CO2��g��?2CO��g����H=+172.5kJ•mol-1��
���ݸ�˹���ɣ���-�ڿɵã�CO��g��+H2O��g��?CO2��g��+H2��g�������ʱ�Ϊ����֮�
��3��������t0ʱ�ı�һ��������ʹ���ߢ������ߢı�����˲��COŨ�Ȳ��䣬ƽ��ʱCO��Ũ�Ȳ��䣬�ı�������Ӱ��ƽ���ƶ���ͬʱ���̵���ƽ���ʱ�䣬�����¶�Ӱ��ƽ���ƶ�����ӦΪ�����������ķ�Ӧ������ѹǿƽ�ⲻ�ƶ�����CO��Ũ�Ȼ�����Ӧ��ʹ�ô�����
������t0ʱ�̽������������ѹ����2L�������������䣩��˲��CO��Ũ�ȱ�Ϊ$\frac{2mol/L��3L}{2L}$=3mol/L������ѹǿ����ƽ�ⲻ�ƶ�����Ӧ��ת���ʲ��䣬��ƽ��ʱCO��Ũ��Ϊ$\frac{3mol/L��3L}{2L}$=4.5mol/L����Ӧ���ʼӿ죬���̵���ƽ��ʱ�䣮
��� �⣺��1����0min��10min�ڵ������ʵ����仯Ϊ0.42mol����v��N2��=$\frac{\frac{0.42mol}{3L}}{10min}$=0.014mol/��L•min����
�ʴ�Ϊ��0.014mol/��L•min����
�ڸ��ݱ������ݿ�֪��20minʱ����ƽ��״̬��ƽ��ʱ������������̼���ʵ�����Ϊ0.6mol��NOΪ0.8mol��ƽ�ⳣ��K=$\frac{c��{N}_{2}����c��C{O}_{2}��}{{c}^{2}��NO��}$=$\frac{\frac{0.6}{3}��\frac{0.6}{3}}{��\frac{0.8}{3}��^{2}}$=0.56��
�ɷ�Ӧǰ����������ʵ������䣬���������ʵ�������Ũ�ȼ���Ũ���̣���Qc=$\frac{0.75��1.08}{1��{2}^{2}}$=0.56=K���ʴ���ƽ��״̬����ʱv������=v���棩��
�ʴ�Ϊ��0.56��=��
��A������v��NO�����棩=2v��N2�����棩����v��NO��������=2v��N2�����棩����v��NO�����棩=v��NO������������Ӧ����ƽ��״̬����A��ȷ��
B�����ڵ����������̼��ѧ������֮��Ϊ1��1��������������������CO2��N2�������ʼ��Ϊ1��1������˵������ƽ�⣬��B����
C����������ܵ����ʵ������䣬�淴Ӧ���л�������������������������ƽ����Է�����������ƽ����Է����������ֲ���˵������ƽ�⣬��C��ȷ��
D����Ӧǰ����������ʵ������䣬������ѹǿʼ�ձ��ֲ��䣬��D����
��ѡ��AC��
��һ���¶��£�NO����ʼŨ������ЧΪ����ѹǿ����Ӧǰ����������ʵ������䣬ƽ�ⲻ�ƶ���NO��ת���ʲ��䣬
�ʴ�Ϊ�����䣻
��2����֪����C��s��+H2O��g��?CO��g��+H2��g����H=+131.3kJ•mol-1��
��C��s��+CO2��g��?2CO��g����H=+172.5kJ•mol-1��
���ݸ�˹���ɣ���-�ڿɵã�CO��g��+H2O��g��?CO2��g��+H2��g�������H=131.3kJ•mol-1-172.5kJ•mol-1=-41.2kJ•mol-1��
�ʴ�Ϊ��-41.2kJ•mol-1��
��3��������t0ʱ�ı�һ��������ʹ���ߢ������ߢı�����˲��COŨ�Ȳ��䣬ƽ��ʱCO��Ũ�Ȳ��䣬�ı�������Ӱ��ƽ���ƶ���ͬʱ���̵���ƽ���ʱ�䣬�����¶�Ӱ��ƽ���ƶ�����ӦΪ�����������ķ�Ӧ������ѹǿƽ�ⲻ�ƶ�����CO��Ũ�Ȼ�����Ӧ��ʹ�ô�����
�ʴ�Ϊ�����������
������t0ʱ�̽������������ѹ����2L�������������䣩��˲��CO��Ũ�ȱ�Ϊ$\frac{2mol/L��3L}{2L}$=3mol/L������ѹǿ����ƽ�ⲻ�ƶ�����Ӧ��ת���ʲ��䣬��ƽ��ʱCO��Ũ��Ϊ$\frac{3mol/L��3L}{2L}$=4.5mol/L����Ӧ���ʼӿ죬���̵���ƽ��ʱ�䣬c��CO���淴Ӧʱ��t�仯������Ϊ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼�黯ѧƽ���йؼ��㡢��ѧƽ��Ӱ�����ء�ƽ��״̬�жϡ�ƽ�ⳣ�����㼰Ӧ�á���Ӧ�ȵļ���ȣ���3������ͼΪ�״��㣬ע��CO��Ũ�����⣬�Ѷ��еȣ�

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�| A�� | ���� | B�� | ���²����� | C�� | ����Ƿ�©ˮ | D�� | ����Ƿ���� |
| A�� | ��Al��Ͷ��NaOH��Һ�У�Al+OH-+H2O�TAlO${\;}_{2}^{-}$+H2�� | |
| B�� | ��KIO3����������Һ�е�KI��5I-+IO3-+3H2O�T3I2+6OH- | |
| C�� | ��CuSO4��Һ�м���Na2O2��Na2O2+2Cu2++2H2O�T2Na++2Cu��OH��2��+O2�� | |
| D�� | ��0.2 mol/L��NH4Al��SO4��2��Һ��0.3 mol/L��Ba��OH��2��Һ�������ϣ�2Al3++3SO42-+3Ba2++6OH-�T2Al��OH��3��+3BaSO4�� |
 ̼�������ǻ�����ѧ���о����ȵ���⣮
̼�������ǻ�����ѧ���о����ȵ���⣮I��ij�о�С���ֽ�����CO��g����H2O��g���Ļ������ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У�һ�������·�����Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g����H��0���õ�������ݣ�
| ʵ���� | �¶�/�� | ��ʼ����mol�� | ƽ������mol�� | �ﵽƽ���� ��Ҫʱ��/min | ||
| CO��g�� | H2O��g�� | CO2��g�� | H2��g�� | |||
| I | 800 | 2 | 2 | x | 1 | 5 |
| II | 900 | 1 | 2 | 0.5 | 0.5 | T1 |
| III | 900 | 2 | 2 | a | a | T2 |
��2���������ж���800��ʵ��������CO��g����H2O��g����Ӧһ���ﵽƽ��״̬����BD��
A��������ѹǿ���ٱ仯 B��n2��H2��=n��H2O��•n��CO��
C����������ܶȲ��� D��������CO��=������CO2��
��3��ʵ��II��III��CO��ƽ��ת���ʣ���II��CO������III��CO�� �����������=����ͬ����T1��T2��a=$\sqrt{3}$-1���ȷ��ֵ����
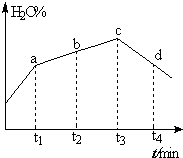
��4����ʵ����������Ϊ�ھ��ȵ��ܱ������н��У�ʵ����H2O��g����ת������ʱ��仯��ʾ��ͼ��ͼ��ʾ��b����������������������=����������t3��t4ʱ�̣�H2O��g����ת����H2O%���͵�ԭ���Ǹ÷�Ӧ�ﵽƽ�����ӦΪ���ȷ�Ӧ�ҷ�Ӧ����Ϊ�������������������¶����ߣ���Ӧ������У�
��5��CO��H2��һ�������ºϳɼ״����״�/��������ȼ�ϵ���У�����32g�״����������ת��4.5mol���ӣ������ĵ缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O���õ���е���Ч��Ϊ75%��������Ч�ʦ�=$\frac{ʵ��ת�Ƶ�����}{����ת�Ƶ�����}$��100%��
| A�� | �÷�Ӧ��ƽ�ⳣ������ʽΪk=$\frac{{c}^{3}��C��•c��D��}{{c}^{2}��A��•{c}^{3}��B��}$ | |
| B�� | �����¶ȣ��÷�Ӧ��ƽ�ⳣ������ | |
| C�� | �ӷ�Ӧ��ʼ10min���÷�Ӧ��ƽ����Ӧ����v��C��Ϊ0.12mol/��L•min�� | |
| D�� | B��ƽ��ת����Ϊ60% |
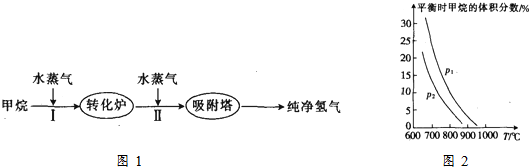
��1����I�����ķ�ӦΪCH4��g��+H2O��g��?CO��g��+3H2��g����
��д���÷�Ӧ��ƽ�ⳣ������ʽ$\frac{c��CO����{c}^{3}��{H}_{2}��}{c��C{H}_{4}����c��{H}_{2}O��}$��
����֪�ڡ�ˮ̼�ȡ�$\frac{c��{H}_{2}O��}{c��C{H}_{4}��}$=3ʱ������¶ȣ�T ����ѹǿ��p����������Ӧ��Ӱ����ͼ2��ʾ���������¶ȣ��÷�Ӧ��ƽ�ⳣ��K�������������С�����䡱������ͼ��֪P1��P2�������������������=����
��2�������ķ�ӦΪCO��g��+H2O��g��?CO2��g��+H2 ��g����T1�¶�ʱ����2L�ĺ����ܱ�������ͨ��һ������CO��H2O��g������Ӧ�����в�ò������������ʾ������t1��t2����
| ��Ӧʱ�䣨min�� | n��CO����mol�� | N��H2O����mol�� |
| 0 | 1.20 | 0.60 |
| t1 | 0.80 | |
| t2 | 0.20 |
�����ﵽƽ����������������䣬ֻ����ԭƽ����ϵ����ͨ��0.20mol H2O��g����������˵����ȷ����ab��
a��CO��ת���ʽ����� b��H2O��g�����������������
c��������ܶȽ����� d����ѧƽ�ⳣ��������
| A�� | �ڳ��³�ѹ�£�11.2 L����������ԭ����ĿΪNA | |
| B�� | 32g��������ԭ����ĿΪNA | |
| C�� | 5.6g�����������ᷴӦ���ɱ�״����H2�����Ϊ2.24L | |
| D�� | 2L 0.1mol•L-1 K2SO4��Һ����������ԼΪ1.4NA |

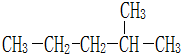 ��2-�����飻
��2-�����飻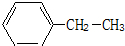 �ұ�
�ұ� 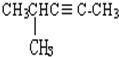 4-��-2-��Ȳ��
4-��-2-��Ȳ��