��Ŀ����
(1997���Ϻ���36)�Ӵ����������ŷŵ�β���к�������SO2��Ϊ��ֹ��Ⱦ���������ŷ�ǰ�跨�����ۺ����á�(1)ij���᳧ÿ���ŷŵ�10000 m3β���к�0.2%(�������)��SO2������NaOH��Һ��ʯ�Ҽ�O2����������Ԫ�ز���ʧ�������Ͽɵõ�����ǧ��ʯ��(CaSO4��2H2O)?
(2)�����һ�������β��ͨ��100 mL 2 mol��L��1��NaOH��Һ��ʹ����ȫ��Ӧ�����ⶨ������Һ��16.7 g���ʡ��Է�������Һ�ijɷ֣�������ȷ�����ɷֵ����ʵ�����
(3)������β��������ʯ��Ĺ����У��м������NaHSO3������β���ŷŵ���������ȡ��SO2��NaOH�����ʵ�������ѱ�ֵ���Ӷ�������������ƵIJ���������n(SO2)��n(NaOH)��n(NaHSO3)�ֱ��ʾSO2��NaOH��NaHSO3�����ʵ�������![]() ����д��x�ڲ�ͬȡֵ��Χʱ��n(NaHSO3)��ֵ��n(NaHSO3)��n(SO2)��n(NaOH)��Ĺ�ϵʽ��
����д��x�ڲ�ͬȡֵ��Χʱ��n(NaHSO3)��ֵ��n(NaHSO3)��n(SO2)��n(NaOH)��Ĺ�ϵʽ��
x | n(NaHSO3) |
|
|
|
|
|
|
�𰸣�
��1��153.6Kg
��2������Һ�ɷ�ΪNa2SO3��NaHSO3 NaHSO30.1Ħ��Na2SO30.05Ħ
��3��
X= |
|
X��1/2 | 0 |
1/2 |
|
X��1 |
|
����Ϻõ������ˡ�����˵������Ҫ���ؿ���ѧ�����ۺ�Ӧ������������ľ���ɫ�������������ɫ�ǣ��������ƶ����ϣ���ѧ�ͻ�ѧ���ۺ�Ӧ�ã���ѧ֪ʶ�뻷�����ϵ����ˣ���ӳ���˸߿���ѧ��������ķ�չ���ƺ��ص㡣����������£�
(1)����ԭ���غ�ɵù�ϵʽ
SO2 �� CaSO4��2H2O
22.4 L 172 g
1��104��103��0.2% L m(CaSO4��2H2O)��10��3
��֮�� m(CaSO4��2H2O)=153.6 kg
(2)Ҫ���ü��˼��跨�����ƶϣ���Һ�п��ܷ���������Ӧ��
SO2+2NaOH====Na2SO3+H2O ��
SO2+NaOH====NaHSO3 ��
���谴��Ӧ�ٽ��У�����ΪNa2SO3��m(Na2SO3)=12.6 g
�谴��Ӧ�ڽ��У�����ΪNaHSO3��m(NaHSO3)=20.8 g
����20.8��16.7��12.6���������ʳɷ�ΪNa2SO3��NaHSO3�Ļ���
��������NaHSO3�����ʵ���Ϊx,Na2SO3�����ʵ���Ϊy
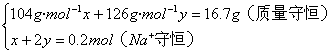
��֮��x=0.1 mol, y=0.05 mol
(3)�ɽ���������з������ۣ�
���ݷ�Ӧ(1)��(2)��ȷ��![]() ��
��![]() ������㡣
������㡣
��Ӧ (1) (1)��(2) (2)
���� 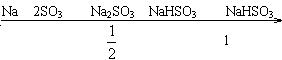
![]() ��ȡֵ
��ȡֵ![]()
![]()
![]()
���۽��Ϊ
X= |
|
X��1/2 | 0 |
1/2 |
|
X��1 |
|
