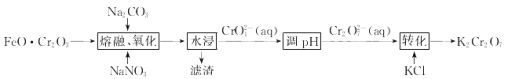
��Ŀ����
����Ŀ�������仯����������������Ӧ�ù㷺���ش��������⣺
(1)���й�����2025�����й�����ʵʩ����ǿ��ս�Ե�һ��ʮ���ж����졣������(CrN)���м��ߵ�Ӳ�Ⱥ���ѧǿ�ȡ�����Ŀ���ʴ���ܺ����ȶ����ܣ����������ִ���ҵ�з��Ӹ���Ҫ�����ã���д��Cr3+����Χ�����Ų�ʽ____����̬������ԭ�ӵĺ���δ�ɶԵ�����֮��Ϊ____��
(2)�������ľ���ṹ�������Ȼ�����ͬ�����������۵�(1282��)���Ȼ��� (801'C)�ĸߣ���Ҫԭ����________��
(3)�������[(NH4)2S2O8]���㷺���������ع�ҵ��ʯ�Ϳ��ɡ����ۼӹ�����֬��ҵ�����ҵ�ȣ����������N��S��O�ĵ�һ�������ɴ�С��˳��Ϊ _______������NH4+�Ŀռ乹��Ϊ____________
(4)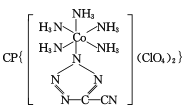 ��20����80����������Ƶĵ��Ͷ۸���ҩ��������
��20����80����������Ƶĵ��Ͷ۸���ҩ��������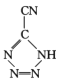 ��[Co(NH3)5H2O](ClO4)3��Ӧ�ϳɵģ�
��[Co(NH3)5H2O](ClO4)3��Ӧ�ϳɵģ� �йµ��Ӷ���������ֵΪ _______�� CP������Co3+����λ��Ϊ ______ ��
�йµ��Ӷ���������ֵΪ _______�� CP������Co3+����λ��Ϊ ______ ��
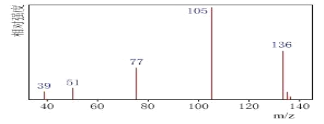
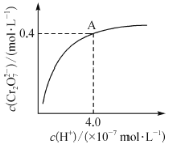
(5)�����������Ǵ��Բ����о��е��ȵ����֮һ��������и߱��ʹŻ�ǿ�ȡ��ͽ�������������ýϸߵ����ŵ��ʣ����м�����г�DZ���������Ӹ�ṹ��ͼ��ʾ����֪�����ܶ�Ϊ��gcm-3�������ӵ�����ΪNA��
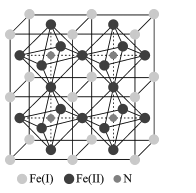
��д�������������Ķѻ���ʽΪ____��
�ڸû�����Ļ�ѧʽΪ ___��
�ۼ���� Fe(II)Χ�ɵİ���������Ϊ____cm3��
���𰸡�3d3 2:1 ����������������������࣬�����ܽϴ� N>O>S �������� 5:4 6 �����������ܶѻ� Fe4N ![]()
��������
(1)��̬Cr�ĺ�������Ų�ʽΪ1s22s22p63s23p63d54s1����̬��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p3��
(2)����ṹ�������Ȼ�����ͬ���������������Խ�࣬����Խ������Խ���۷е�Խ�ߣ�
(3)ͬ����Ԫ�أ���һ��������˵�������������ͬ����Ԫ�أ���һ��������˵�����������С����Ԫ�����������Ų�����ȫ����������ȫ��ʱ�ṹ�ȶ�����һ�����ܱ�����Ԫ�ظߣ������ӻ������жϿռ乹�ͣ�
(4)˫���к���һ��������һ��������������һ���������������������ݽṹ��ʽ��Ԫ��������������Ԫ�صļ�����ʽ�жϹµ��Ӷ����������������������ֱ������������ĸ�����Ϊ��λ����
(5) ������ͼʾ�����������λ��Ϊ��ԭ�ӣ�
�ڸ���ͼʾ��һ�������е�ԭ�Ӹ����þ�̯�����м��㣻
�۸�����=![]() �Ƶ����㾧���ⳤ���ٸ�����������Ľṹ�ص���������
�Ƶ����㾧���ⳤ���ٸ�����������Ľṹ�ص���������
(1)��̬Cr�ĺ�������Ų�ʽΪ1s22s22p63s23p63d54s1����6��δ�ɶԵĵ��ӣ���̬Cr3+�ĺ�������Ų�ʽΪ1s22s22p63s23p63d3��Cr3+����Χ�����Ų�ʽ3d3����̬��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p3��3��δ�ɶԵĵ��ӣ���̬������ԭ�ӵĺ���δ�ɶԵ�����֮��Ϊ2:1��
(2)����ṹ�������Ȼ�����ͬ���������������Խ�࣬����Խ������Խ���۷е�Խ�ߣ�����������������������࣬�����ܽϴʵ������۵���Ȼ��Ƶĸߣ�
(3)ͬ����Ԫ�أ���һ��������˵�����������С����S��Oͬ����Ԫ�أ���һ��������˵�������������Ԫ�����������Ų�����ȫ����������ȫ��ʱ�ṹ�ȶ�����һ�����ܱ�����Ԫ�ظߣ���̬��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p3�����ȶ�״̬�����һ������N��O����һ�������ɴ�С��˳��ΪN��O��S��NH4+������ԭ��ΪN����۲���Ӷ���=4+![]() ��(5-1-4��1)=4��Ϊsp3�ӻ����ռ乹��Ϊ�������壻
��(5-1-4��1)=4��Ϊsp3�ӻ����ռ乹��Ϊ�������壻
(4)˫���к���һ��������һ��������������һ������������������ �У���һ��-C��N������2������������˫���зֱ���һ�����������ݽṹ�м�����ʽ��̼ԭ��û�й¶Ե��ӣ�ÿ����ԭ����һ�Թ¶Ե��ӣ��µ��Ӷ���������ֵΪ5:4�������������������ֱ������������ĸ�����Ϊ��λ����CP������Co3+����λ��Ϊ6��
�У���һ��-C��N������2������������˫���зֱ���һ�����������ݽṹ�м�����ʽ��̼ԭ��û�й¶Ե��ӣ�ÿ����ԭ����һ�Թ¶Ե��ӣ��µ��Ӷ���������ֵΪ5:4�������������������ֱ������������ĸ�����Ϊ��λ����CP������Co3+����λ��Ϊ6��
(5)�ٸ���ͼʾ�����������λ��Ϊ��ԭ�ӣ������������Ķѻ���ʽΪ�����������ܶѻ���
�ڸ���ͼʾ��һ�������е���ԭ��Ϊ��������ģ�����=8��![]() +6��
+6��![]() =4����ԭ��λ�ھ����ڲ���N����Ϊ1����û�����Ļ�ѧʽΪFe4N��
=4����ԭ��λ�ھ����ڲ���N����Ϊ1����û�����Ļ�ѧʽΪFe4N��
�۸�����=![]() ���������V=
���������V=![]() �������ⳤ=
�������ⳤ=![]() =
= =
=![]() ��Fe(II)Χ�ɵİ������У��ⳤ=
��Fe(II)Χ�ɵİ������У��ⳤ=![]() ��
��![]() ����������һ������=
����������һ������=![]() ��(
��(![]() ��
��![]() )2��
)2��![]() ��
��![]() =
=![]() ������������Ϊ=2��
������������Ϊ=2��![]() =
=![]() ��
��
![]()

����Ŀ������ʵ�鲻�ܴﵽԤ��ʵ��Ŀ���ǣ���
��� | ʵ������ | ʵ��Ŀ�� |
A | �����£���pH��ֽ�ⶨŨ��Ϊ0.1mol��L-1NaClO��Һ��0.1mol��L-1CH3COONa��Һ��pH | �Ƚ�HClO��CH3COOH������ǿ�� |
B | ��ʢ��1mL��������Һ���Թ��еμ�NaCl��Һ���������г������ɣ��������еμ�Na2S��Һ | ˵��һ�ֳ�����ת��Ϊ��һ���ܽ�ȸ�С�ij��� |
C | ��������FeCl3��MgCl2��Һ�м�������Mg(OH)2��ĩ������һ����� | ��ȥMgCl2������FeCl3 |
D | �����£��ֱ���2֧�Թ��м�����ͬ�������ͬŨ�ȵ�Na2S2O3��Һ���ٷֱ������ͬ�����ͬŨ�ȵ�ϡ���� | �о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� |
A. A B. B C. C D. D