��Ŀ����
A~F��ΪԪ�����ڱ���ǰ������Ԫ�أ��������Ϣ���±���
��ش��������⣺
(1)D�ļ۵��ӵĵ����Ų�ʽ�� ��Fԭ�ӵ�ԭ�ӽṹʾ��ͼΪ ��
(2)A��B�ĵ�һ�����ܵĴ�С˳��Ϊ ��
(3)AB3����Aԭ�ӵ��ӻ��������Ϊ_____����A2B��Ϊ�ȵ�����ķ��ӵķ���ʽΪ (��дһ������)��
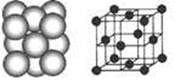
(4) D����ľ�����ͼ��ʾΪ�����������ܶѻ�(�ھ����Ķ�������ľ�����һ��Dԭ��)����D�ľ�����Dԭ�ӵ���λ��Ϊ ��
(5)��֪17gA�ļ��⻯�������������̬ˮʱ�ų�QkJ����������д��A�ļ��⻯����������Ȼ�ѧ��Ӧ����ʽ ��
(6)C2B2�ĵ���ʽΪ____��������E�Ķ��Ȼ�����Һ��Ӧ������Ӧ��C2B2��E�Ķ��Ȼ�������ʵ���֮��Ϊ1��2����÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
| Ԫ�� | �� �� �� Ϣ |
| A | A�Ļ�̬ԭ�����������Ų�ʽΪ2s22p3 |
| B | B�ǵؿ��к�����ߵ�Ԫ�� |
| C | C����B�ļ����ӵĵ��Ӳ�ṹ��ͬ |
| D | D��һ�ֺ��ص�������Ϊ64��������Ϊ35 |
| E ��F | E ��F��ͬ������ͬ�壬��ԭ������F��E��2 |
��ش��������⣺
(1)D�ļ۵��ӵĵ����Ų�ʽ�� ��Fԭ�ӵ�ԭ�ӽṹʾ��ͼΪ ��
(2)A��B�ĵ�һ�����ܵĴ�С˳��Ϊ ��
(3)AB3����Aԭ�ӵ��ӻ��������Ϊ_____����A2B��Ϊ�ȵ�����ķ��ӵķ���ʽΪ (��дһ������)��
(4) D����ľ�����ͼ��ʾΪ�����������ܶѻ�(�ھ����Ķ�������ľ�����һ��Dԭ��)����D�ľ�����Dԭ�ӵ���λ��Ϊ ��

(5)��֪17gA�ļ��⻯�������������̬ˮʱ�ų�QkJ����������д��A�ļ��⻯����������Ȼ�ѧ��Ӧ����ʽ ��
(6)C2B2�ĵ���ʽΪ____��������E�Ķ��Ȼ�����Һ��Ӧ������Ӧ��C2B2��E�Ķ��Ȼ�������ʵ���֮��Ϊ1��2����÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
(1)3d104s1 
(2)N����O
(3)sp2 CO2
(4)12
(5)4NH3(g)+5O2(g)=4NO(g)+6H2O(g) ��H="-Q" kJ/mol
(5) 3Na2O2+6FeCl2+6H2O=6NaCl+4Fe(OH)3��+2FeCl3
3Na2O2+6FeCl2+6H2O=6NaCl+4Fe(OH)3��+2FeCl3

(2)N����O
(3)sp2 CO2
(4)12
(5)4NH3(g)+5O2(g)=4NO(g)+6H2O(g) ��H="-Q" kJ/mol
(5)
 3Na2O2+6FeCl2+6H2O=6NaCl+4Fe(OH)3��+2FeCl3
3Na2O2+6FeCl2+6H2O=6NaCl+4Fe(OH)3��+2FeCl3����֪��A�Ļ�̬ԭ�����������Ų�ʽΪ2s22p3��A��NԪ�أ�B�ǵؿ��к�����ߵ�Ԫ�أ�B��O��C����B�ļ����ӵĵ��Ӳ�ṹ��ͬ��C��Na��D��һ�ֺ��ص�������Ϊ64��������Ϊ35��D��Cu��E ��F��ͬ������ͬ�壬���������ǵ������ڢ�����ԭ������F��E��2��E��F�ֱ���Fe��Ni��
(1)Cu�ļ۵��ӳ���4s�ϵ����⣬������3d�ϵĵ��ӣ�
(2)N��2p���ڰ����״����N��һ�����ܴ���O��
(3)AB3-��N�ŵ��ӶԶ���=(5+1-3��2)/2=0������ȡsp3����N2O��Ϊ�ȵ�������CO2��
(4)ͬһ�������ϵĶԽ��߶���4������3���棬��12����
(1)Cu�ļ۵��ӳ���4s�ϵ����⣬������3d�ϵĵ��ӣ�
(2)N��2p���ڰ����״����N��һ�����ܴ���O��
(3)AB3-��N�ŵ��ӶԶ���=(5+1-3��2)/2=0������ȡsp3����N2O��Ϊ�ȵ�������CO2��
(4)ͬһ�������ϵĶԽ��߶���4������3���棬��12����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ



