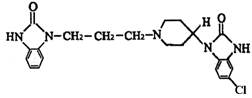
��Ŀ����
�ο����Т١�����������⡣
������ֵ��ʹ 1 g ��֬��������Ҫ���������صĺ�������
�ڵ�ֵ��ʹ 100 g ��֬�ӳ�ʱ���ĵ��ʵ�Ŀ�����
�۸�����֬������ֵ����ֵ�б����£�
(1)������(C17H33COO)3C3H5(��Է�������884)�γɵ��ͣ���������������ʱ������ֵΪ_________��д���䷴Ӧ����ʽ____________________��(2)�����Т١��۵ĺ����������ʵ��Ĵʾ䡣
���������ͱȻ����������е�___________�ࣻ
�ڻ��ͱ�ţ��������__________�ࣻ
��Ӳ�����͵�ֵ��С��ԭ����______________________________��
(3)Ϊʹ��ֵΪ 180 �� 100 g ����Ӳ�������������������ڱ�״����Ϊ_______L��
(4)�����нṹʽ������������������ֵΪ 430���� n Ϊ���٣���������·�Ӧ����ʽ
CnH2n+1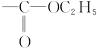 +KOH
+KOH ( )+( )
( )+( )
������ֵ��ʹ 1 g ��֬��������Ҫ���������صĺ�������
�ڵ�ֵ��ʹ 100 g ��֬�ӳ�ʱ���ĵ��ʵ�Ŀ�����
�۸�����֬������ֵ����ֵ�б����£�
| | ������ | �������� | ţ�� | ���� | Ӳ������ | ���� |
| ����ֵ | 190 | 180 | 192 | 226 | 193 | 193 |
| ��ֵ | 90 | 182 | 38 | 38 | 5 | 126 |
���������ͱȻ����������е�___________�ࣻ
�ڻ��ͱ�ţ��������__________�ࣻ
��Ӳ�����͵�ֵ��С��ԭ����______________________________��
(3)Ϊʹ��ֵΪ 180 �� 100 g ����Ӳ�������������������ڱ�״����Ϊ_______L��
(4)�����нṹʽ������������������ֵΪ 430���� n Ϊ���٣���������·�Ӧ����ʽ
CnH2n+1
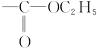 +KOH
+KOH ( )+( )
( )+( )(1)190 mg (C17H33COO)3C3H5+3KOH 3C17H33COOK+C3H5(OH)3
3C17H33COOK+C3H5(OH)3
(2)�ٲ��������� �ڵͼ�֬����ijɷ� ��������̼̼˫���� (3)15.9
(4)C4H9COOC2H5+KOH C4H9COOK+C2H5OH
C4H9COOK+C2H5OH
 3C17H33COOK+C3H5(OH)3
3C17H33COOK+C3H5(OH)3(2)�ٲ��������� �ڵͼ�֬����ijɷ� ��������̼̼˫���� (3)15.9
(4)C4H9COOC2H5+KOH
 C4H9COOK+C2H5OH
C4H9COOK+C2H5OH(1)(C17H33COO)3C3H5+3KOH 3C17H33COOK+C3H5(OH)3
3C17H33COOK+C3H5(OH)3
884 g 3��56��103 mg
1 g x
x=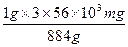 ="190" mg
="190" mg
���Ըõ����͵�����ֵΪ190��
(3)254 g I2�� 2 g H2�����ʵ��������� H2�ڱ�״���µ����Ϊ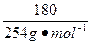 ��22.4 L��mol-1 =" 15.9" L��
��22.4 L��mol-1 =" 15.9" L��
(4)CnH2n+1COOC2H5����Է�������Ϊ14n+74��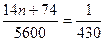
��� n=4
C4H9COOC2H5+KOH C4H9COOK + C2H5OH
C4H9COOK + C2H5OH
 3C17H33COOK+C3H5(OH)3
3C17H33COOK+C3H5(OH)3884 g 3��56��103 mg
1 g x
x=
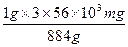 ="190" mg
="190" mg���Ըõ����͵�����ֵΪ190��
(3)254 g I2�� 2 g H2�����ʵ��������� H2�ڱ�״���µ����Ϊ
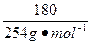 ��22.4 L��mol-1 =" 15.9" L��
��22.4 L��mol-1 =" 15.9" L��(4)CnH2n+1COOC2H5����Է�������Ϊ14n+74��
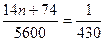
��� n=4
C4H9COOC2H5+KOH
 C4H9COOK + C2H5OH
C4H9COOK + C2H5OH
��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ
 3B+C������������C�к���3���ǻ�����Է�������Ϊ92��B��������ʵ�����NaOHǡ����ȫ�к͡���A��C�Ľṹ��ʽΪA__________��C__________��
3B+C������������C�к���3���ǻ�����Է�������Ϊ92��B��������ʵ�����NaOHǡ����ȫ�к͡���A��C�Ľṹ��ʽΪA__________��C__________��