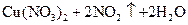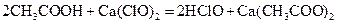��Ŀ����
��CH4����ԭNO2�������������������Ⱦ�����磺
��CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H=-574kJ��mol-1
��CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2��g����H=-1160kJ��mol-1
����˵������ȷ���� �� ��
��CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H=-574kJ��mol-1
��CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2��g����H=-1160kJ��mol-1
����˵������ȷ���� �� ��
| A���ɷ�Ӧ�ٿ���֪��CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��L����H>-574kJ��mol-1 |
| B�������ʵ�����CH4�μӷ�Ӧʱ���ų�������Ϊ17.34kJ |
| C������0.2moLCH4��ԭNO2��N2���ų�������Ϊ17.34kJ |
| D������0.02moLCH4��ԭNO2��N2������������ת�Ƶĵ�������Ϊ0.16mol |
A
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 MnCl2+Cl2��+2H2O���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ ,�뽫�û�ѧ����ʽ��дΪ���ӷ���ʽ______________________________�������Ĺ�ҵ�Ʒ��ǵ�ⱥ��ʳ��ˮ�����оٳ������������������е�Ӧ�ã�������_________________��___________________��
MnCl2+Cl2��+2H2O���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ ,�뽫�û�ѧ����ʽ��дΪ���ӷ���ʽ______________________________�������Ĺ�ҵ�Ʒ��ǵ�ⱥ��ʳ��ˮ�����оٳ������������������е�Ӧ�ã�������_________________��___________________��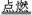 2CO2
2CO2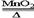 2KCl+3O2��
2KCl+3O2�� Fe2O3 +3H2O
Fe2O3 +3H2O ��ԭ��Ӧ
��ԭ��Ӧ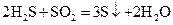

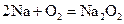
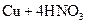 ��Ũ��=
��Ũ��=