��Ŀ����
FeSO4?7H2O�㷺����ҽҩ��ҵ����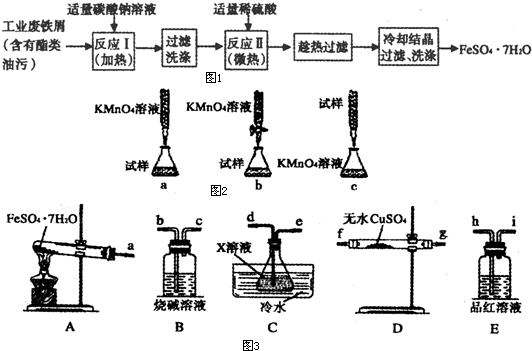
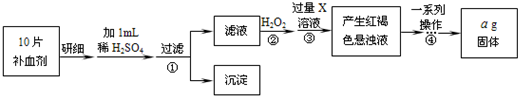
��1����ͼ1���Թ�ҵ����мΪԭ������FeSO4?7H2O������ͼ��
����д���пհף�
�ټ�����̼������Һ��Ŀ����
���жϷ�Ӧ����ɵ�������
�۲ⶨFeSO4?7H2O��Ʒ��Fe2+�����ķ�������KMnO4��Һ�ζ�����5Fe2++Mn
| O | - 4 |
���ȡ2.8500g FeSO4?7H2O��Ʒ�����Ƴ�250mL��Һ��
����ȡ25.00mL������Һ����ƿ�У�
���������ữ��0.01000moL/L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
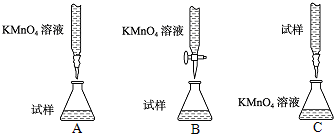
ijͬѧ�����ͼ2��ʾ�ĵζ���ʽ�У����������
��2����֪FeSO4?7H2O�����ڼ��������·������·�Ӧ��2FeSO4?7H2O
| ||
����д���пհף�
������������˳��Ϊa��
��װ��C�е�XΪ
��������1��������̼������Һ��ˮ���Լ��ԣ������ڼ�����Һ��ˮ��Ϊ����ˮ�����ʳ�ȥ��ˮ�ⷴӦ�����ȷ�Ӧ�����ȴٽ�ˮ�⣬������ǿ��
�ڷ�Ӧ����ɵ������Ǽ���ϡ���ᣬ���岻���ܽ��ҹ����������������֤����Ӧ��ȫ��������Һ���Ʋ��������Ҫ��������
�۵ζ����������ø��������Һ�ζ������ⶨ���������������Һ��ǿ��������Ҫ����ʽ�ζ����н��еζ�ʵ������жϣ���Ӧ�յ������ø��������Һ����ɫָʾ��Ӧ�յ㣬���ݷ�Ӧ�����ӷ���ʽ������Ԫ�����ʵ����õ���Ԫ������������ע����Һ����仯���ⶨ���ƫ�ͣ�������ʵ��������������ӱ�����������Ʒ�к������������ʣ�
��2���ٷֽ����ɵ��ж����������������ˮ����������ͼ3װ�ÿɼ���÷�Ӧ��������������Dװ�ü������ɵ�ˮ������ͨ��Cװ����ȴ��Ϊ��������������ͨ��װ��E���������������Ĵ��ڣ����ʣ������ͨ��Bװ������β����
��װ��C�����Ȼ�����Һ����ˮ�����ǽ�������������������գ�
�ڷ�Ӧ����ɵ������Ǽ���ϡ���ᣬ���岻���ܽ��ҹ����������������֤����Ӧ��ȫ��������Һ���Ʋ��������Ҫ��������
�۵ζ����������ø��������Һ�ζ������ⶨ���������������Һ��ǿ��������Ҫ����ʽ�ζ����н��еζ�ʵ������жϣ���Ӧ�յ������ø��������Һ����ɫָʾ��Ӧ�յ㣬���ݷ�Ӧ�����ӷ���ʽ������Ԫ�����ʵ����õ���Ԫ������������ע����Һ����仯���ⶨ���ƫ�ͣ�������ʵ��������������ӱ�����������Ʒ�к������������ʣ�
��2���ٷֽ����ɵ��ж����������������ˮ����������ͼ3װ�ÿɼ���÷�Ӧ��������������Dװ�ü������ɵ�ˮ������ͨ��Cװ����ȴ��Ϊ��������������ͨ��װ��E���������������Ĵ��ڣ����ʣ������ͨ��Bװ������β����
��װ��C�����Ȼ�����Һ����ˮ�����ǽ�������������������գ�
����⣺��1����̼������Һˮ��ɼ��ԣ�������̼������Һ����֬�ᷢ��ˮ����������ˮ�����ʳ�ȥ����ӦI��Ҫ���������ӣ����ȴٽ�̼������ӵ�ˮ�������ǿȥ��������ǿ���ʴ�Ϊ����ȥ���ۣ����´ٽ�ˮ�⣬������ǿ��ȥ����������ǿ��
���жϷ�Ӧ����ɵ������ǣ��Ǽ���ϡ���ᣬ���岻���ܽ��ҹ����������������֤����Ӧ��ȫ����Ӧ����Ҫ100mL1mol/L��ϡ���ᣬ��98.3%����=1.84g/cm3��Ũ�������ƣ����õ���������Ͳ���ձ�������������ͷ�ιܼ�100ml����ƿ��
�ʴ�Ϊ�����岻���ܽ⣬�������������ð����100ml����ƿ��
�۵ζ����������ø��������Һ�ζ������ⶨ���������������Һ��ǿ��������Ҫ����ʽ�ζ����н��еζ�ʵ�飬ѡ��b����Ӧ�յ������ø��������Һ����ɫָʾ��Ӧ�յ㣬�μ����һ�θ��������Һʱ����Һ�仯Ϊdz��ɫ�Ұ���Ӳ��仯˵����Ӧ�ﵽ�յ㣻
25ml��Һ�����������Һ�ζ�����Ӧ����0.01000moL/L KMnO4��Һ20.00mL���ʵ���=0.01000mol/L��0.0200L=2��10-4mol��
5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
5 1
n��Fe2+�� 2��10-4mol
n��Fe2+��=10-3mol
250ml��Һ��n��Fe2+��=10-2mol
��Ʒ��FeSO4?7H2O����������=
=0.975��
�������������ⶨ����Ʒ��FeSO4?7H2O����������ƫ�ͣ��ⶨ�����в��������ɺ��ԣ���������ʵ��������������ӱ�����������Ʒ�к������������ʵȣ�
�ʴ�Ϊ��b���μ����һ�θ��������Һʱ����Һ�仯Ϊdz��ɫ�Ұ���Ӳ��仯��0.975����Ʒ�к������������ʣ���Ʒ���ֱ�������
��2���ٷֽ����ɵ��ж����������������ˮ����������ͼ3װ�ÿɼ���÷�Ӧ��������������Dװ�ü������ɵ�ˮ������ͨ��Cװ����ȴ��Ϊ��������������ͨ��װ��E���������������Ĵ��ڣ����ʣ������ͨ��Bװ������β��������������˳��Ϊa��f��g��d��e��h��i��b��
�ʴ�Ϊ��f��g��d��e��h��i��b��
��װ��C�е�XΪ��������������Ȼ�����Һ����װ������ˮ�������ǣ�SO3��H2O��Ӧ�Ƿ��ȷ�Ӧ�������¶�������SO3��ˮ���գ�
�ʴ�Ϊ���Ȼ�����Һ��SO3��H2O��Ӧ�Ƿ��ȷ�Ӧ�������¶�������SO3��ˮ���գ�
���жϷ�Ӧ����ɵ������ǣ��Ǽ���ϡ���ᣬ���岻���ܽ��ҹ����������������֤����Ӧ��ȫ����Ӧ����Ҫ100mL1mol/L��ϡ���ᣬ��98.3%����=1.84g/cm3��Ũ�������ƣ����õ���������Ͳ���ձ�������������ͷ�ιܼ�100ml����ƿ��
�ʴ�Ϊ�����岻���ܽ⣬�������������ð����100ml����ƿ��
�۵ζ����������ø��������Һ�ζ������ⶨ���������������Һ��ǿ��������Ҫ����ʽ�ζ����н��еζ�ʵ�飬ѡ��b����Ӧ�յ������ø��������Һ����ɫָʾ��Ӧ�յ㣬�μ����һ�θ��������Һʱ����Һ�仯Ϊdz��ɫ�Ұ���Ӳ��仯˵����Ӧ�ﵽ�յ㣻
25ml��Һ�����������Һ�ζ�����Ӧ����0.01000moL/L KMnO4��Һ20.00mL���ʵ���=0.01000mol/L��0.0200L=2��10-4mol��
5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
5 1
n��Fe2+�� 2��10-4mol
n��Fe2+��=10-3mol
250ml��Һ��n��Fe2+��=10-2mol
��Ʒ��FeSO4?7H2O����������=
| 0.01mol��278g/mol |
| 2.8500g |
�������������ⶨ����Ʒ��FeSO4?7H2O����������ƫ�ͣ��ⶨ�����в��������ɺ��ԣ���������ʵ��������������ӱ�����������Ʒ�к������������ʵȣ�
�ʴ�Ϊ��b���μ����һ�θ��������Һʱ����Һ�仯Ϊdz��ɫ�Ұ���Ӳ��仯��0.975����Ʒ�к������������ʣ���Ʒ���ֱ�������
��2���ٷֽ����ɵ��ж����������������ˮ����������ͼ3װ�ÿɼ���÷�Ӧ��������������Dװ�ü������ɵ�ˮ������ͨ��Cװ����ȴ��Ϊ��������������ͨ��װ��E���������������Ĵ��ڣ����ʣ������ͨ��Bװ������β��������������˳��Ϊa��f��g��d��e��h��i��b��
�ʴ�Ϊ��f��g��d��e��h��i��b��
��װ��C�е�XΪ��������������Ȼ�����Һ����װ������ˮ�������ǣ�SO3��H2O��Ӧ�Ƿ��ȷ�Ӧ�������¶�������SO3��ˮ���գ�
�ʴ�Ϊ���Ȼ�����Һ��SO3��H2O��Ӧ�Ƿ��ȷ�Ӧ�������¶�������SO3��ˮ���գ�
���������⿼�����������ʵķ��������ʺ�����ʵ����֤������ʵ����̷����������������ʺͻ����ǹؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д�
�����Ŀ