��Ŀ����
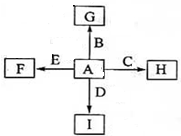 ��ͼ�У�A��B��C��D��E�dz������ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĻ������֪���ٷ�Ӧ2C+G
��ͼ�У�A��B��C��D��E�dz������ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĻ������֪���ٷ�Ӧ2C+G
| ||
| ||
�ش����⣺
��1�����з�Ӧ�Ļ�ѧ����ʽΪ
��2������A�ķ���ʽΪ
��3��C��NaOH��Һ��Ӧ�����ӷ���ʽΪ
������A��B��C��D��E�dz������ʣ��ɢٷ�Ӧ2C+G
2B+H�����ų��������ȣ��÷�Ӧ����������ĺ��ӣ���CΪAl��GΪFe2O3��BΪFe��HΪAl2O3��A+C��H������AΪO2��I��һ�ֳ������������壬IΪCO2������E���Է�����Ӧ��2E+I
2F+D��F��EԪ�ص���������Ϊ60%����FΪMgO��EΪMg��DΪC��������ʵ����������
| ||
| ||
����⣺A��B��C��D��E�dz������ʣ��ɢٷ�Ӧ2C+G
2B+H�����ų��������ȣ��÷�Ӧ����������ĺ��ӣ���CΪAl��GΪFe2O3��BΪFe��HΪAl2O3��A+C��H������AΪO2��I��һ�ֳ������������壬IΪCO2������E���Է�����Ӧ��2E+I
2F+D��F��EԪ�ص���������Ϊ60%����FΪMgO��EΪMg��DΪC��
��1�������з�Ӧ�Ļ�ѧ����ʽΪ2Al+Fe2O3
2Fe+Al2O3���ʴ�Ϊ��2Al+Fe2O3
2Fe+Al2O3��
��2��������������֪��AΪO2��IΪCO2���ʴ�Ϊ��O2��CO2��
��3��C��NaOH��Һ��Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
| ||
| ||
��1�������з�Ӧ�Ļ�ѧ����ʽΪ2Al+Fe2O3
| ||
| ||
��2��������������֪��AΪO2��IΪCO2���ʴ�Ϊ��O2��CO2��
��3��C��NaOH��Һ��Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
���������⿼��������ƶϣ���ȷ��Ӧ��Ϊ���ȷ�Ӧ����Ӧ��ΪMg�������̼�ķ�Ӧ�ǽ�����ͻ�ƿڣ���Ϥ���ʵ����ʼ��ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
�����Ŀ
 ��ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪���ٷ�ӦC+G
��ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪���ٷ�ӦC+G
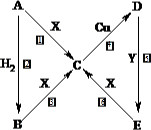

 B��H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
B��H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ� 2F��D��F��EԪ�ص���������Ϊ60%���ش����⣺
2F��D��F��EԪ�ص���������Ϊ60%���ش����⣺ ��ͼ�У�A��B��C��D��E�dz������ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĻ������֪���ٷ�Ӧ2C+G
��ͼ�У�A��B��C��D��E�dz������ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĻ������֪���ٷ�Ӧ2C+G 2B+H�����ų��������ȣ��÷�Ӧ����������ĺ��ӣ���I��һ�ֳ������������壬����E���Է�����Ӧ��2E+I
2B+H�����ų��������ȣ��÷�Ӧ����������ĺ��ӣ���I��һ�ֳ������������壬����E���Է�����Ӧ��2E+I 2F+D��F��EԪ�ص���������Ϊ60%��
2F+D��F��EԪ�ص���������Ϊ60%��