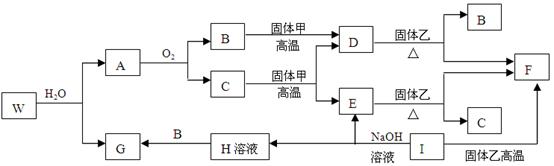
��Ŀ����
��֪����ס�E��I��F��Ϊ�����ĵ��ʣ�����E�ڳ�����Ϊ��̬��GΪ��ɫ��״�����������ܽ������ᣬ�����ܽ�������������Һ��A�ڳ�����Ϊ��̬����������ȫ��Ӧʱ���������1:2��W�������ִ��ڲ�ͬ�����ڵ�Ԫ����ɵĻ������ˮ��Ӧ����A��Gʱ�Ļ�ѧ������֮��Ϊ1:3:3:1��������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�IJ���δȫ����ʾ�����Իش��������⣺

��1��B�ĵ���ʽΪ �������ҵĻ�ѧʽ������ ��
��2������ת���У���C��E ��D��F ��E��F ��I��F ��I��E ��A��B ������
�û���Ӧ���ǣ�����ţ� ��
��3��д��W��ˮ��Ӧ�Ļ�ѧ����ʽ_____________________________________________,
I��NaOH��Һ��Ӧ�����ӷ���ʽ__________________________________________��
��1��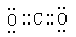 Fe2O3
��2���٢ۢ�
Fe2O3
��2���٢ۢ�
��3��Al(CH3)3+3H2O=3CH4��+Al(OH)3 �� Al+2OH-+2H2O=2AlO2-+3H2��
��������GΪ��ɫ��״��������Gˮ��������������ת����A�����ʿ��ƣ�A�Ǽ��飬B��CO2��C��ˮ������̼��E��������I������H��ƫ�����ƣ��ҿ�������������F����������W�����Ԫ�أ��Լ���ˮ��Ӧ����A��Gʱ�Ļ�ѧ������֮��Ϊ1:3:3:1��֪��W��Al(CH3)3+3H2O=3CH4��+Al(OH)3 ��