��Ŀ����
��ͼ��һ���绯ѧ���̵�ʾ��ͼ����ش��������⣺
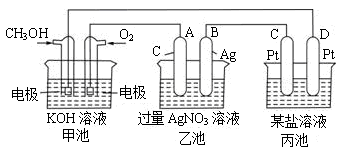
��1��ͼ�м׳ص����� ______ (�ԭ��ء������ء���Ƴء�)��
��2��д��ͨ��CH3OH�ĵ缫�ĵ缫��Ӧʽ��__________��
��3���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ__________�����ҳ���B������������5.4gʱ���׳�������������O2�����Ϊ ____L(��״����)����ʱ������_____�缫���C����D��������1.6gij����������ص�ij����Һ������ ____ (�����) A��MgSO4��Һ B��CuSO4��Һ C��NaCl��Һ D��AgNO3��Һ
��2��д��ͨ��CH3OH�ĵ缫�ĵ缫��Ӧʽ��__________��
��3���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ__________�����ҳ���B������������5.4gʱ���׳�������������O2�����Ϊ ____L(��״����)����ʱ������_____�缫���C����D��������1.6gij����������ص�ij����Һ������ ____ (�����) A��MgSO4��Һ B��CuSO4��Һ C��NaCl��Һ D��AgNO3��Һ
��1��ԭ���
��2 ��CH3OH ��8OH�D�D6e����CO32 ����6H2O
��3 ��4AgNO3��2H2O 4Ag��O2����4HNO3; 0.28 ��D �� BD
4Ag��O2����4HNO3; 0.28 ��D �� BD
��2 ��CH3OH ��8OH�D�D6e����CO32 ����6H2O
��3 ��4AgNO3��2H2O
 4Ag��O2����4HNO3; 0.28 ��D �� BD
4Ag��O2����4HNO3; 0.28 ��D �� BD
��ϰ��ϵ�д�
�����Ŀ

 Ϊ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH
Ϊ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH 2K2CO3+6H2O
2K2CO3+6H2O
 _________L����״���£���
_________L����״���£���