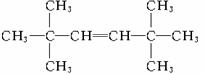��Ŀ����
��1��ij��A����Է�������Ϊ128����A�ķ���ʽΪ
 ��
��
��2��0.2molij��B����������ȫȼ�պ�����CO2��H2O��1.2mol���Իش�
��B�ķ���ʽΪ
����B����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣���B�Ľṹ��ʽΪ
 ��
��
����B��ʹ��ˮ��ɫ�ҷ���������̼ԭ�Ӵ���ͬһƽ�棬��д��B�Ľṹ��ʽ����ϵͳ����������֮��
C9H20
C9H20
��C10H8
C10H8
����AΪ��������������A�Ľṹ��ʽΪ

��2��0.2molij��B����������ȫȼ�պ�����CO2��H2O��1.2mol���Իش�
��B�ķ���ʽΪ
C6H12
C6H12
������B����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣���B�Ľṹ��ʽΪ


����B��ʹ��ˮ��ɫ�ҷ���������̼ԭ�Ӵ���ͬһƽ�棬��д��B�Ľṹ��ʽ����ϵͳ����������֮��
��������1�������ķ���ʽΪCxHy��������Է�������Ϊ128����C��Hԭ��Ϊ�������㣻
��2������0.2molij��B����������ȫȼ�պ�����CO2��H2O��1.2mol����֪1mol���к���6molCԭ�ӣ�12molHԭ�ӣ��Դ˿ɵó�����ʽ�����������ƶϿ��ܵĽṹ��ʽ��
��2������0.2molij��B����������ȫȼ�պ�����CO2��H2O��1.2mol����֪1mol���к���6molCԭ�ӣ�12molHԭ�ӣ��Դ˿ɵó�����ʽ�����������ƶϿ��ܵĽṹ��ʽ��
����⣺��1��������ֻ��C��H����Ԫ������Է�������Ϊ128���������ΪCxHy����12x+y=128������x��yȡֵӦΪ����������ϡ�C�ļۡ�ԭ��֪x=10��=8����x=9��y=20����������壬���Կ��ܵķ���ʽΪC9H20��C10H8����AΪ��������������A�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ��C9H20��C10H8�� ��
��
��2����0.2molij��B����������ȫȼ�պ�����CO2��H2O��1.2mol����֪1mol���к���6molCԭ�ӣ�12molHԭ�ӣ�
�����ʽΪC6H12���ʴ�Ϊ��C6H12��
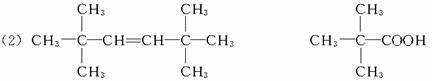
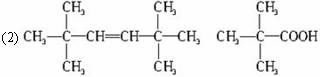
����B����ʹ��ˮ��ɫ��˵�������в���˫��������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�˵���ṹ�Գƣ�ӦΪ�����飬�ṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
����B��ʹ��ˮ��ɫ�ҷ���������̼ԭ�Ӵ���ͬһƽ�棬˵�������к���C=C�������ҽṹ�Գƣ�ӦΪ2��3-����-2-��ϩ������CH3��2C=C��CH3��2��
��B�Ľṹ��ʽΪ��CH3��2C=C��CH3��2 Ϊ2��3-����-2-��ϩ��
 ��
���ʴ�Ϊ��C9H20��C10H8��
 ��
����2����0.2molij��B����������ȫȼ�պ�����CO2��H2O��1.2mol����֪1mol���к���6molCԭ�ӣ�12molHԭ�ӣ�
�����ʽΪC6H12���ʴ�Ϊ��C6H12��
����B����ʹ��ˮ��ɫ��˵�������в���˫��������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�˵���ṹ�Գƣ�ӦΪ�����飬�ṹ��ʽΪ
 ��
���ʴ�Ϊ��
 ��
������B��ʹ��ˮ��ɫ�ҷ���������̼ԭ�Ӵ���ͬһƽ�棬˵�������к���C=C�������ҽṹ�Գƣ�ӦΪ2��3-����-2-��ϩ������CH3��2C=C��CH3��2��
��B�Ľṹ��ʽΪ��CH3��2C=C��CH3��2 Ϊ2��3-����-2-��ϩ��
���������⿼���л������ʽ�ͽṹ��ʽ��ȷ������Ŀ�ѶȲ���ע���ƶ��л������ʽ�ĽǶȣ��������ʵ������ƶϿ��ܵĽṹ�ص㣮

��ϰ��ϵ�д�
�����Ŀ
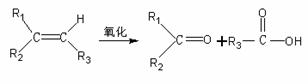
ij��A����Է���������140������C������������0.857��A������������Cԭ�Ӳ���Hֱ��������A��һ��������ֻ��������G��G����ʯ����Һ��졣
 |
��֪��
���
��1�� A�ķ���ʽ______________________
��2�� ������A��G�Ľṹ��ʽ�ֱ��ǣ�
��3�� _________ _____��___________________.
��4�� ��Gͬ���ͬ���칹�壨����G��������_______��.
 ��������������⣺
��������������⣺