��Ŀ����
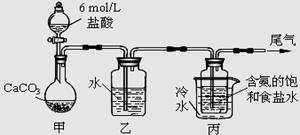
��.ʵ����������500mL 0.2mol/L NaCl��Һ
��1����ʵ���õ�����������ҩ�ס��ձ�����Ͳ��������ƽ(���롢����)������������ͷ�ιܣ���ȱ�ٵIJ��������� ��
��2������ʱ�����ȡNaCl�������� g
��3�������ƹ����У�������������ȷ������£����в����ᵼ�������Ƶ���ҺŨ��ƫ�ߵ��� ����ѡ����ţ�
��û��ϴ���ձ��Ͳ�����
�ڼ�����ˮʱ�����������˿̶ȣ�ȡ������ˮʹҺ��ǡ�õ��̶���
������ƿ�����������������ˮ
�ܶ���ʱ���ӱ���
�ݶ���ʱ���ӱ���
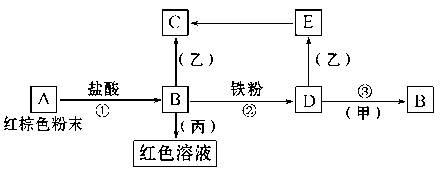
��.��10�֣�ij����Ϊ���������һ��ѧ��ȤС����ʵ������Ըô�������Ԫ�صļ�̬����̽���������й����ϵ�֪Fe2+�ܱ�����KMnO4��Һ������ʹ����KMnO4��Һ��ɫ��HNO3����ǿ�����ԡ�����ɶ���Ԫ�ؼ�̬��̽����
��1������������衣
����1����������Ԫ��Ϊ+3�ۣ�
����2�� ��
����3����������Ԫ�ؼ���+3������+2��
��2�����ʵ�鷽����
��3�����ݣ�2����ʵ�鷽������ʵ�飺
����1��ȡһ������ϡ�������Թ��У�������������ӣ�Ŀ���� ��
����2��ȡ������Ʒ���Թ��У����봦���������Ტ���ȣ��õ�A��Һ��
����3��ȡA��Һ���Թܣ��μ�KSCN��Һ������������Ѫ��ɫ������� ������������Ѫ��ɫ�������1��3������
����4��Ϊ�˽�һ��ȷ������3���Ǽ���1���Ǽ���3��������ȡA��Һ���Թܣ�����KMnO4��Һ���������� ���������1��������֮�������3������
��4����˼
ijͬѧ������2�е�ϡ����ij�ϡ����õ���A��Һ����A��Һ�еμ�KSCN��Һ�����Ѫ��ɫ���ɴ˵ó��ô�������Ԫ��Ϊ+3�۵Ľ��ۡ����жϸý����Ƿ����
���������������������
��1��500 mL����ƿ��û��д����ƿ���ݻ�������ƿ��д�������÷֣���2�֣�
��2��5.9g��5.85�����÷֣���2�֣�
��3���ݣ�2�֣�
��1����������Ԫ��Ϊ+2�ۣ�2�֣�
��3����ȥ�������ܽ��������2�֣�����3��2��2�֣� ����4�����������Һ����ɫ��2�֣�
��4����������2�֣�
���������������1��������Һ����IJ�����������ƿ����ע������ƿ����ע�������500 mL����ƿ����2������500mL 0.2mol/L NaCl��Һ��������NaCl5.85 g������������ƽֻ�ܳ�����0.1g�����Ա���д��5.9g����3����ƫ�͢�ƫ�͢���Ӱ���ƫ�͢�ƫ��
��.��1�����������ļ���1��3��֪������2Ӧ���Ǵ�������Ԫ��Ϊ+2��
��3����������Ҳ���������ԣ��������������ӣ����Լ�����������ӵ�Ŀ���dz�ȥ�������ܽ��������
����3�������������ܺ�KSCN��Һ��Ӧ�Ժ�ɫ��������������Ѫ��ɫ�������2������������Ѫ��ɫ�������1��3������
����4���������Ӿ��л�ԭ�ԣ��ܱ����Ը��������Һ��������ɫ������������ָ��������Һ����ɫ���������1��������֮�������3������
��4������������������ԣ����������ὫFe2+������Fe3+���Ӷ�����ʵ����۲�ȷ���ʲ�������
���㣺������������������Ԫ�ػ��ϼ�̽����ʵ���ж�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����X����������������ͭ��ɣ�ȡ������ȵ�������������ͼ����ʵ�飺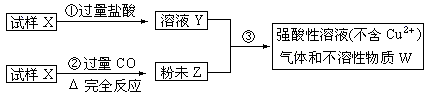
��1����д�����ۢ��з�����ȫ����Ӧ�����ӷ���ʽ��____________________________________��
��2��Ҫʹ����Xת��Ϊ��ĩZ������CO�⣬������ʹ�� ��
| A������ | B����̿ | C������ | D������ |

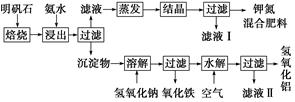

 NaHCO3��+NH4Cl������ĸҺ�����ַ�����
NaHCO3��+NH4Cl������ĸҺ�����ַ�����