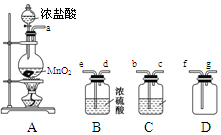
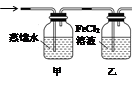
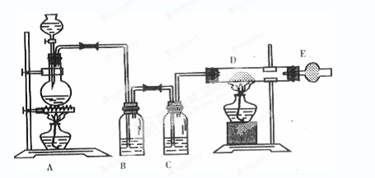
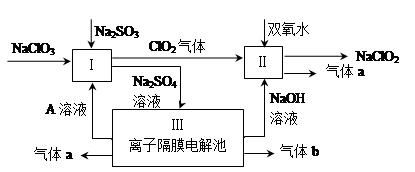��Ŀ����
��X��Y��Z����Ԫ�أ���֪����X��Y��Z�ĵ����ڳ����¾�Ϊ���壻��X���ʿ���Z������ȼ�գ�������̬XZ����XZ��������ˮ�������X+��Z������ˮ��Һ��ʹ��ɫʯ����ֽ��죻��ÿ2��X2���ӿ���1��Y2���ӻ�������2��X2Y���ӣ�X2Y�ڳ�����Ϊ��ɫҺ�壻��Z��������X2Y�У�������Һ����Ư�����á�
��1���ƶ�X��Y����Ԫ�أ�X________��Y_________����Ԫ�����ƣ���
��2��д���ݹ����еĻ�ѧ��Ӧ�ķ���ʽ ��
��3��д����ҵ����ȡZ���ʵĻ�ѧ����ʽ ��
��4��дZ������ʯ���鷴Ӧ�Ļ�ѧ����ʽ ��
��1���ƶ�X��Y����Ԫ�أ�X________��Y_________����Ԫ�����ƣ���
��2��д���ݹ����еĻ�ѧ��Ӧ�ķ���ʽ ��
��3��д����ҵ����ȡZ���ʵĻ�ѧ����ʽ ��
��4��дZ������ʯ���鷴Ӧ�Ļ�ѧ����ʽ ��
��1���� �� ��2��Cl+H2O HCl+HClO
HCl+HClO
��3��2NaCl��2H2O 2NaOH��H2����Cl2�� ��4��2Ca(OH)2 +2Cl2��Ca(ClO)2+CaCl2+2H2O
2NaOH��H2����Cl2�� ��4��2Ca(OH)2 +2Cl2��Ca(ClO)2+CaCl2+2H2O
 HCl+HClO
HCl+HClO��3��2NaCl��2H2O
 2NaOH��H2����Cl2�� ��4��2Ca(OH)2 +2Cl2��Ca(ClO)2+CaCl2+2H2O
2NaOH��H2����Cl2�� ��4��2Ca(OH)2 +2Cl2��Ca(ClO)2+CaCl2+2H2O���������X�ĵ�����Z�ĵ�����ȼ�գ�����XZ������XZ��������ˮ����ˮ��Һ�е����X+��Z-��XZ��ˮ��Һ��ʹʯ����Һ��죬˵��XΪH2��ZΪCl2��XZΪHCl��������X�ĵ��ʿ���һ����Y�ĵ��ʻ�������������X2Y��X2Y������ΪҺ�壬˵��YΪO2��X2YΪH2O��Cl2����H2O�У���Ӧ����HCl��HClO��HClO����Ư�����á�
��1��ͨ�����Ϸ���֪��X��YԪ�����Ʒֱ����⡢�����ʴ�Ϊ���⣻����
��2��Cl2����H2O�У���Ӧ����HCl��HClO����Ӧ�Ļ�ѧ����ʽΪCl+H2O
 HCl+HClO��
HCl+HClO����3����ҵ���õ�ⱥ���Ȼ�����Һ�ķ�����ȡ��������Ӧ����ʽΪ2NaCl��2H2O
 2NaOH��H2����Cl2����
2NaOH��H2����Cl2������4���������������Ʒ�Ӧ�����Ȼ��ơ�������ƺ�ˮ����Ӧ����ʽΪ2Ca(OH)2��2Cl2��Ca(ClO)2��CaCl2��2H2O

��ϰ��ϵ�д�
�����Ŀ