��Ŀ����
�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ����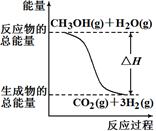
��CH3OH(g)��H2O(g)��CO2(g)��3H2(g) ?��H ����49.0 kJ/mol
��CH3OH(g)��1/2O2(g)��CO2(g)��2H2(g) ?��H����192.9 kJ/mol
����������Ӧ������˵����ȷ����( )
| A����Ӧ���е������仯����ͼ��ʾ |
| B��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| C��1 mol CH3OH���ȼ�շų�������Ϊ192.9 kJ |
| D������֪2H2(g)��O2(g)��2H2O(g)??H����483.8 kJ/mol |
D
�������������A����Ӧ��Ϊ���ȷ�Ӧ��ͼ���ʾ���Ƿ��ȷ�Ӧ������B����Ӧ��CH3OHת��ΪH2��Ϊ���ȷ�Ӧ������C����Ӧ�ڵIJ���ΪH2���� CH3OH���ȼ��Ӧ������H2O������1 mol CH3OH���ȼ�շų�����������192.9 kJ������D�����ݸ�˹���ɣ�2H2(g)��O2(g)��2H2O(g) ��?H=-2?H1+2?H2=��483.8 kJ?mol?1����ȷ��
���㣺���⿼�黯ѧ��Ӧ����ЧӦ����˹���ɵ�Ӧ�á�

ij��Ӧ�ķ�Ӧ�����������仯����ͼ��ʾ��ͼ��E1��ʾ����Ӧ�Ļ�ܣ�E2��ʾ�淴Ӧ�Ļ�ܣ����Ը÷�Ӧ���й�������ȷ����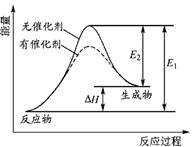
| A���÷�Ӧ������ӦΪ���ȷ�Ӧ |
| B�������ܸı䷴Ӧ���ʱ� |
| C���������ܽ��ͷ�Ӧ�Ļ�� |
| D���淴Ӧ�Ļ�ܴ�������Ӧ�Ļ�� |
��֪1g������ȫȼ������ˮ����ʱ�ų�����121kJ����������1molO=O����ȫ����ʱ��������496 kJ��ˮ����1molH-O���γ�ʱ�ų�����463 kJ����������1molH-H������ʱ��������Ϊ
| A��920 kJ | B��557 kJ | C��436 kJ | D��188 kJ |
����˵������ȷ����(����)
| A��úת��Ϊú����ȼ�գ��������ȼ��Ч�� |
| B������������ͨ�����ѵȹ�����ʵ�С��������������á���Դ���� |
| C����ʯȼ����ȼ�չ������ܲ�����Ⱦ������CO��SO2���к����� |
| D����Ȼ�������壬�������ڻ�ʯȼ�� |
ͨ�����ǰѲ�1 molij��ѧ�������ĵ��������ɸû�ѧ���ļ��ܡ����ܵĴ�С���Ժ�����ѧ����ǿ����Ҳ�����ڹ��㻯ѧ��Ӧ�ķ�Ӧ�ȣ���H������ѧ��Ӧ�ġ�H���ڷ�Ӧ����ܼ�������������ܼ���֮�
| ��ѧ�� | Si��O | Si��Cl | H��H | H��Cl | Si��Si | Si��C |
| ����/kJ��mol��1 | 460 | 360 | 436 | 431 | 176 | 347 |
��ҵ�ϸߴ����ͨ�����з�Ӧ��ȡ��SiCl4��g����2H2��g��
 Si��s����4HCl��g�����÷�Ӧ�ķ�Ӧ�ȡ�HΪ�� ��
Si��s����4HCl��g�����÷�Ӧ�ķ�Ӧ�ȡ�HΪ�� ��A����412 kJ��mol��1 B����412 kJ��mol��1
C����236 kJ��mol��1 D����236 kJ��mol��1
����˵����ȷ����
| A����101kPaʱ��1molC������O2��Ӧ����1molCOʱ���ų�110.5kJ��������C��ȼ����Ϊ110.5kJ/mol |
| B����101kPaʱ��1molH2��ȫȼ������Һ̬ˮ���ų�285.8kJ������H2ȼ����Ϊ ��285.8kJ/mol |
| C���ⶨHCl��NaOH��Ӧ���к���ʱ��ÿ��ʵ���Ӧ����3���¶ȣ���������ʼ�¶ȡ�NaOH��ʼ�¶Ⱥͷ�Ӧ����ֹ�¶� |
| D����ϡ��Һ�У�H��(aq) �� OH--��aq) ="==" H2O��l)����H= ��57.3kJ/mol��������0.5molH2SO4��Ũ�����뺬1molNaOH����Һ��ϣ��ų�����������57.3kJ |
��Ϣ�����ϡ���Դ����Ϊ�¿Ƽ������ġ�����֧���������й۵�������������
| A���ڼ�������������Դʱ��,���ܡ�̫���ܡ����ܽ���Ϊ��Ҫ��Դ |
| B���Ӵ�ú̿�Ŀ����ٶ�,����ú̿ȼ�ϵĹ�Ӧ��,�Ի���ʯ��Σ�� |
| C����������Ϣ��ҵ���й㷺Ӧ��,������µ���Ҫ�����Ƕ������� |
| D�����½ṹ�մɵ����裨Si3N4�����нϸߵ�Ӳ�Ⱥ���ĥ��,�������������������� |
���������Ȼ�ѧ����ʽ��
��1��C(s)��O2(g)=CO2(g)����H1����393.5 kJ��mol��1
��2��H2(g)��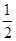 O2(g)=H2O(l) ��H2����285.8 kJ��mol��1
O2(g)=H2O(l) ��H2����285.8 kJ��mol��1
��3��CH3COOH(l)��2O2(g)=2CO2(g)��2H2O(l) ��H3����870.3 kJ��mol��1
���Լ����2C(s)��2H2(g)��O2(g)=CH3COOH(l)�ķ�Ӧ��Ϊ (����)
| A����H����244.1 kJ��mol��1 |
| B����H����488.3 kJ��mol��1 |
| C����H����996.6 kJ��mol��1 |
| D����H����996.6 kJ��mol��1 |
