��Ŀ����
ij�л���A�����ĺ��������Ϊ�ⶨ�����������ʵ������
����һ���¶Ⱥ�ѹǿ�½�A��������������ͬ�¡�ͬѹ������������76����
�ڳ�ȡ7.6g�л���A���ڹ�����������ȫȼ�գ�����Ӧ��Ļ������ͨ������ŨH2SO4�������Ϊ10.64L��Ũ��������3.6g���ٽ����µ�����ͨ��ʢ��������ʯ�ҵĸ���ܣ��������������1.68L������������ڱ�״���²ⶨ����
��1��ȷ��A�ķ���ʽ��
��2����A������FeCl3��Һ����ɫ��Ӧ����ȷ��A�Ľṹ��ʽ��дһ�ּ��ɣ���
����һ���¶Ⱥ�ѹǿ�½�A��������������ͬ�¡�ͬѹ������������76����
�ڳ�ȡ7.6g�л���A���ڹ�����������ȫȼ�գ�����Ӧ��Ļ������ͨ������ŨH2SO4�������Ϊ10.64L��Ũ��������3.6g���ٽ����µ�����ͨ��ʢ��������ʯ�ҵĸ���ܣ��������������1.68L������������ڱ�״���²ⶨ����
��1��ȷ��A�ķ���ʽ��
��2����A������FeCl3��Һ����ɫ��Ӧ����ȷ��A�Ľṹ��ʽ��дһ�ּ��ɣ���
��������һ���¶Ⱥ�ѹǿ�½�A��������������ͬ�¡�ͬѹ������������76������A����Է���������152��7.6g�л���A�����ʵ�����0.05mol��Ũ��������3.6g����Ӧ�����ɵ�ˮ��������3.6g�����ʵ�������0.2mol���ٽ����µ�����ͨ��ʢ��������ʯ�ҵĸ���ܣ��������������1.68L������������ڱ�״���²ⶨ�������Է�Ӧ�����ɵ�CO2��10.64L-1.68L=8.96L�����ʵ�����8.96L��22.4L/mol=0.04mol�������ԭ���غ��֪��A��̼��ԭ�ӵĸ����ֱ���8��8��������ԭ�ӵĸ�����
=3����˻�ѧʽ��C8H8O3��
��A������FeCl3��Һ����ɫ��Ӧ��˵������������ǻ���1molAֻ����1molNaOH��Ӧ��1molA�����������Ʒ�Ӧ������1molH2��˵�������к����ǻ����Ȼ����Դ˽����⣮
| 152-96-8 |
| 16 |
��A������FeCl3��Һ����ɫ��Ӧ��˵������������ǻ���1molAֻ����1molNaOH��Ӧ��1molA�����������Ʒ�Ӧ������1molH2��˵�������к����ǻ����Ȼ����Դ˽����⣮
����⣺��1��Mr��A��=76��Mr��H2��=76��2=152��
Ũ��������3.6g����Ӧ�����ɵ�ˮ��������3.6g��
n��H��=
=0.4mol��
�ٽ����µ�����ͨ��ʢ��������ʯ�ҵĸ���ܣ��������������1.68L������������ڱ�״���²ⶨ�������Է�Ӧ�����ɵ�CO2��10.64L-1.68L=8.96L��
n��C��=
=0.4mol��
�����ԭ���غ��֪��
n��O��=
=0.15mol��
n��C����n��H����n��O��=0.4mol��0.4mol��0.15mol=8��8��3��
A��ʵ��ʽ��C8H8O3��ʽ��Ϊ152����ʵ��ʽ���Ƿ���ʽ��������ʽΪC8H8O3��
��A�ķ���ʽΪC8H8O3��
��2����A������FeCl3��Һ����ɫ��Ӧ��˵������������ǻ���1molAֻ����1molNaOH��Ӧ��1molA�����������Ʒ�Ӧ������1molH2��˵�������к����ǻ����Ȼ������ܵĽṹ��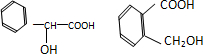 ���ڡ��䡢�Ծ��ɣ���
���ڡ��䡢�Ծ��ɣ���
��A�Ľṹ��ʽΪ �ȣ�
�ȣ�
Ũ��������3.6g����Ӧ�����ɵ�ˮ��������3.6g��
n��H��=
| 3.6g��2 |
| 18g/mol |
�ٽ����µ�����ͨ��ʢ��������ʯ�ҵĸ���ܣ��������������1.68L������������ڱ�״���²ⶨ�������Է�Ӧ�����ɵ�CO2��10.64L-1.68L=8.96L��
n��C��=
| 10.64L-1.68L |
| 22.4L/mol |
�����ԭ���غ��֪��
n��O��=
| 7.6g-0.4mol��1g/mol-0.4mol��12g/mol |
| 16g/mol |
n��C����n��H����n��O��=0.4mol��0.4mol��0.15mol=8��8��3��
A��ʵ��ʽ��C8H8O3��ʽ��Ϊ152����ʵ��ʽ���Ƿ���ʽ��������ʽΪC8H8O3��
��A�ķ���ʽΪC8H8O3��
��2����A������FeCl3��Һ����ɫ��Ӧ��˵������������ǻ���1molAֻ����1molNaOH��Ӧ��1molA�����������Ʒ�Ӧ������1molH2��˵�������к����ǻ����Ȼ������ܵĽṹ��
 ���ڡ��䡢�Ծ��ɣ���
���ڡ��䡢�Ծ��ɣ�����A�Ľṹ��ʽΪ
 �ȣ�
�ȣ����������⿼���л�����ƶϣ������ڻ�ѧʽ���ṹ��ʽȷ�����йؼ�����жϣ��߿��еij������ͣ������е��Ѷȵ����⣮�����ۺ���ǿ�������߿�����ע�ض�ѧ������֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ��������������ּ������ѧ�����������ɡ��ܽ����������������������ѧ���������������ʹ���˼ά����������Ĺؼ������ú��غ㷨���������غ��ԭ���غ�ȣ�

��ϰ��ϵ�д�
 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����Ŀ
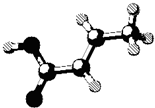 ij�л���ķ��ӽṹ��ͼ��ͼ�й���ʾ������˫�������������������ĺ�����������ڸ��л����������ȷ���ǣ�������
ij�л���ķ��ӽṹ��ͼ��ͼ�й���ʾ������˫�������������������ĺ�����������ڸ��л����������ȷ���ǣ�������| A�����л���Ļ�ѧʽΪC6H6 | B�����л���ɷ���ȡ����Ӧ�ͼӳɷ�Ӧ | C�����л����ʹ��ˮ��ɫ����������NaOH��Һ��Ӧ | D�����л����������ͬϵ�� |


