��Ŀ����
10����Cu��Cu2O��CuO��ɵĻ�����У�����1L0.6mol•L-1HNO3��Һǡ��ʹ������ܽ⣬ͬʱ�ռ���2240mL NO���壨��״����������˵���в���ȷ���ǣ���֪��Cu2O+2H+=Cu+Cu2++H2O����������| A�� | �����������Ե����������������Ե����������ʵ���֮��Ϊ��5��1 | |
| B�� | ���������������������H2���Ȼ�ԭ�����õ����������Ϊ32g | |
| C�� | Cu2O��ϡ���ᷴӦ�����ӷ���ʽΪ��3Cu2O+14H++2NO${\;}_{3}^{-}$�T6Cu2++2NO��+7H2O | |
| D�� | ��������к�0.1mol Cu�����û������ϡ�����ַ�Ӧ����������H2SO4�����ʵ���Ϊ0.1mol |
���� A����Ӧ�����У�����ԭ��NO�����ʵ���Ϊ���������Ե����ᣬʣ�ಿ��Ϊ�������Ե����
B��Cu��Cu2O��CuO��ɵĻ������HNO3��Һǡ�÷�Ӧ������Cu��NO3��2��NO������NԪ���غ����n[Cu��NO3��2]���ٸ���CuԪ���غ���������������ɵ�n��Cu��������m=nM���㻹ԭ�õ�Cu��������
C��ϡ�������ǿ�����ԡ����ԣ���Cu2O��Ӧ��������ͭ��NO��ˮ��
D�����NO����������ݵ���ת���غ����������n��Cu2O�����ٽ�ϣ�2����CuԪ�����ʵ�������������n��CuO����Cu����ϡH2SO4��Ӧ��Cu2O��CuO��ϡH2SO4��Ӧ������Cu2O��CuO����ԭ����H2SO4��H+�������H2O���ݴ˼����������ᣮ
��� �⣺A�������2.24LNO�����ʵ���Ϊ��$\frac{2.24L}{22.4L/mol}$=0.1mol����ԭ���������Ե���������ʵ���Ϊ0.1mol���μӷ�Ӧ������������ʵ���Ϊ��0.6mol/L��1L=0.6mol������Nԭ���غ㣬�������Ե���������ʵ���Ϊ��0.6mol-0.1mol=0.5mol�������������Ե����������������Ե����������ʵ���֮��Ϊ��0.5mol��0.1mol=5��1����A��ȷ��
B��Cu��Cu2O��CuO��HNO3ǡ����ȫ��Ӧʱ����Cu��NO3��2��NO��H2O����������ʵ���0.6mol��NO�����ʵ���=0.1mol������Nԭ���غ��֪��n[Cu��NO3��2]=$\frac{0.6mol-0.1mol}{2}$=0.25mol���������������CuԪ�ع���0.25mol������ͭԪ���غ��֪����H2��ԭCu��Cu2O��CuO������Ӧ�õ�0.25mol Cu���ʹ��������Ϊ��0.25mol��64g/mol=16g����B����
C��Cu2O��ϡHNO3����ΪCu2+��NO3-����ԭΪNO��ͬʱ����H2O���䷴Ӧ�����ӷ���ʽΪ��3Cu2O+14H++2NO3-�T6Cu2++2NO��+7H2O����C��ȷ��
D�����ݵ���ת���غ㣬�������n��Cu2O��=$\frac{0.1mol��3-0.1mol��2}{2}$=0.05mol���ٽ��B��CuԪ�����ʵ�������Cu�غ��֪��n��CuO��=0.25mol-0.1mol-0.05mol��2=0.05mol��������У�0.1mol Cu����ϡH2SO4��Ӧ��0.05molCu2O��0.05molCuO��ϡH2SO4��Ӧ������Cu2O��CuO����ԭ����H2SO4�����H+����H2O���ɵ����ĵ�n��H2SO4��=0.05mol+0.05mol=0.1mol����D��ȷ��
��ѡB��
���� ���⿼������ļ��㡢������ԭ��Ӧ���㣬��Ŀ�Ѷ��еȣ����ض�ѧ��˼ά�����Ŀ��飬B��ע������غ�˼����м��㣬D�����÷�Ӧ����ˮ������H��Oԭ�ӹ�ϵ���㣬�ɱ������÷���ʽ�ķ�����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | OH- | B�� | SO${\;}_{4}^{2-}$ | C�� | Ba2+ | D�� | Cu2+ |
| A�� | ���ὺ�壺OH-��K+��S2-��Na+ | B�� | ������C2H4��CO��SO2��NO | ||
| C�� | ������Һ��Na+��K+��NO3-��NH3•H2O | D�� | ���������Һ��H+��Na+��SO42-��Fe2+ |
| A�� | C8H10 | B�� | CH4 | C�� | CH3CH2OH | D�� | C5H10 |
| A�� | CH2=CH-CH=CH2 | B�� | CH2=CH-C��CH | C�� | 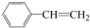 | D�� | 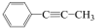 |
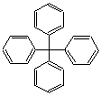
| A�� | ���������ڷ����� | B�� | ����̼ԭ�ӿ�����ͬһƽ���� | ||
| C�� | �˷��ӵ�һ��ȡ��������12�� | D�� | �����ʷ���ʽΪC25H20 |
| A�� | 1 mol����-CH3�������ĵ�������Ϊ9 NA | |
| B�� | ��״���£�22.4L�ȷ������Ĺ��ۼ�Ϊ4NA | |
| C�� | ���³�ѹ�£�142g C10H22�к����ۼ�����ĿΪ31NA | |
| D�� | ��״���£�22.4L���������ļ��Թ��ۼ���ĿΪ4NA |
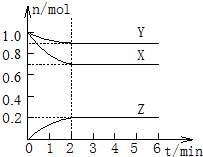 ��һ���¶��£����Ϊ2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ����������գ�
��һ���¶��£����Ϊ2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ����������գ�