��Ŀ����
��12�֣���ĩ״��ƷA���ɵ����ʵ�����MgO��Al2O3�볣������������B��ɵĻ���A������ͼ��ʾ��ת����ϵ��
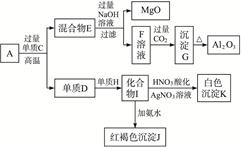
������������⣺
(1)����C��H�Ļ�ѧʽ�ֱ���__________��__________��
(2)д����I����J�����ӷ���ʽ��_____________��
(3)д����F����G�����ӷ���ʽ��________��
(4)��10.7 g��ƷA��MgO��Al2O3��B�����ʵ�����Ϊ0.05 mol����B�Ļ�ѧʽΪ_________��
(5)��B�н���ԭ������ԭ�ӵ�������֮��Ϊ2��3��ȡ7.10 g��ƷA��ǡ���뺬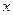 mol HCl��������ȫ��Ӧ����
mol HCl��������ȫ��Ӧ����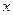 ��ȡֵ��Χ��___________(����С�������λ)��
��ȡֵ��Χ��___________(����С�������λ)��

������������⣺
(1)����C��H�Ļ�ѧʽ�ֱ���__________��__________��
(2)д����I����J�����ӷ���ʽ��_____________��
(3)д����F����G�����ӷ���ʽ��________��
(4)��10.7 g��ƷA��MgO��Al2O3��B�����ʵ�����Ϊ0.05 mol����B�Ļ�ѧʽΪ_________��
(5)��B�н���ԭ������ԭ�ӵ�������֮��Ϊ2��3��ȡ7.10 g��ƷA��ǡ���뺬
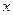 mol HCl��������ȫ��Ӧ����
mol HCl��������ȫ��Ӧ����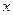 ��ȡֵ��Χ��___________(����С�������λ)��
��ȡֵ��Χ��___________(����С�������λ)��(1)Al��Cl2
(2) ====
====
(3) ====
====
(4)FeO
(5)0.26<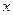 <0.40(����0.27<
<0.40(����0.27<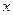 <0.40�����۷֣�
<0.40�����۷֣�
(2)
 ====
====
(3)
 ====
====
(4)FeO
(5)0.26<
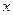 <0.40(����0.27<
<0.40(����0.27<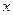 <0.40�����۷֣�
<0.40�����۷֣���������ת����ϵ��֪����DΪ�����ʣ�ԭ�����к��������������Ӧ�ĵ���CΪ�����ʡ�

��ϰ��ϵ�д�
�����Ŀ
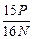 ��100%
��100%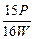 ��100%
��100%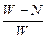 ��100%
��100% ��100%
��100% 2Fe+3CO2������3 mol e-ת�ƣ�����_______mol �����ɣ���_______��CO���Ӳμӷ�Ӧ��
2Fe+3CO2������3 mol e-ת�ƣ�����_______mol �����ɣ���_______��CO���Ӳμӷ�Ӧ��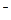 ����ACl2��Ħ�������� ��A�����ԭ�������� ��ACl2�Ļ�ѧʽ�� ��
����ACl2��Ħ�������� ��A�����ԭ�������� ��ACl2�Ļ�ѧʽ�� ��