��Ŀ����
��12�֣�
��1����״���£����ԼΪ11.2 L��NH3��Լ���� �����ӡ����� �����ӡ�
��2��ͬ��ͬѹ�£�ͬ�����İ������������壨H2S���������Ϊ ��ͬ��ͬѹ�£���������ԭ������ȣ����ǵ������Ϊ ��
��3����ij�¶�ʱ��һ������Ԫ��A���⻯��AH3��һ������ܱ������п���ȫ�ֽ��������̬���ʣ���ʱ�ܱ���������������ܵ����ʵ���������75%����A���ʵ�һ����������_____��Aԭ�ӣ�AH3�ֽⷴӦ�Ļ�ѧ����ʽΪ___________________��
��1����״���£����ԼΪ11.2 L��NH3��Լ���� �����ӡ����� �����ӡ�
��2��ͬ��ͬѹ�£�ͬ�����İ������������壨H2S���������Ϊ ��ͬ��ͬѹ�£���������ԭ������ȣ����ǵ������Ϊ ��
��3����ij�¶�ʱ��һ������Ԫ��A���⻯��AH3��һ������ܱ������п���ȫ�ֽ��������̬���ʣ���ʱ�ܱ���������������ܵ����ʵ���������75%����A���ʵ�һ����������_____��Aԭ�ӣ�AH3�ֽⷴӦ�Ļ�ѧ����ʽΪ___________________��
��1��3.01��1023��2�֣� 3.01��1024��2�֣�
��2��2�U1��2�֣� 2�U3��2�֣�
��3��4��2�֣� 4AH3 = A4+6H2��2�֣�
��2��2�U1��2�֣� 2�U3��2�֣�
��3��4��2�֣� 4AH3 = A4+6H2��2�֣�
�������ʵ������йؼ��㡣
��1�����ݱ�״���µ�����Ħ�������֪��11.2L���������ʵ�����11.2L��22.4L/mol��0.5mol����˺��еķ�������10��0.5mol��6.02��1023/mol��3.01��1023�����������к���10�����ӣ����Ժ��е���������0.5mol��6.02��1023/mol��3.01��1024��
��2��ͬ�����İ������������壨H2S�������ʵ���֮����34�U17��2�U1�����Ը��ݰ����ӵ����ɿ�֪�����ߵ����֮����2�U1�����ݶ��ߵĻ�ѧʽ��֪��ͬ��ͬѹ�£���������ԭ������ȣ����ǵ������Ϊ1/3�U1/2��2�U3��
��3�����⻯��ֽ�ķ���ʽ��2xAH3 ="2" Ax+3xH2�������ܱ���������������ܵ����ʵ���������75%��������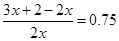 �����x��4����A���ʵ�һ����������4��ԭ�ӣ���Ӧ�ķ���ʽ��4AH3 = A4+6H2��
�����x��4����A���ʵ�һ����������4��ԭ�ӣ���Ӧ�ķ���ʽ��4AH3 = A4+6H2��
��1�����ݱ�״���µ�����Ħ�������֪��11.2L���������ʵ�����11.2L��22.4L/mol��0.5mol����˺��еķ�������10��0.5mol��6.02��1023/mol��3.01��1023�����������к���10�����ӣ����Ժ��е���������0.5mol��6.02��1023/mol��3.01��1024��
��2��ͬ�����İ������������壨H2S�������ʵ���֮����34�U17��2�U1�����Ը��ݰ����ӵ����ɿ�֪�����ߵ����֮����2�U1�����ݶ��ߵĻ�ѧʽ��֪��ͬ��ͬѹ�£���������ԭ������ȣ����ǵ������Ϊ1/3�U1/2��2�U3��
��3�����⻯��ֽ�ķ���ʽ��2xAH3 ="2" Ax+3xH2�������ܱ���������������ܵ����ʵ���������75%��������
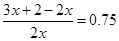 �����x��4����A���ʵ�һ����������4��ԭ�ӣ���Ӧ�ķ���ʽ��4AH3 = A4+6H2��
�����x��4����A���ʵ�һ����������4��ԭ�ӣ���Ӧ�ķ���ʽ��4AH3 = A4+6H2��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ