��Ŀ����
��6�֣���֪����ˮ����ˮ�ܽ�Fe2+������Fe3+������ˮ���ܣ���Fe3+����ʹKI-������Һ����ɫ��
����100mL FeBr2��Һ��ͨ���������Ϊ3.36L��Cl2����ַ�Ӧ������Һ��Cl-��Br-�����ʵ���Ũ����ȣ���ԭFeBr2��Һ�����ʵ���Ũ��Ϊ ��
����1 molFeI2��2mol FeBr2��Һ��ͨ��3 mol Cl2����Һ�������������ӵĸ���֮���� ��
����a molFeI2��bmol FeBr2��Һ��ͨ��c mol Cl2����Ҫ��֤��Һ�к������������ӣ�c��ȡֵ��Χ�� ��
����100mL FeBr2��Һ��ͨ���������Ϊ3.36L��Cl2����ַ�Ӧ������Һ��Cl-��Br-�����ʵ���Ũ����ȣ���ԭFeBr2��Һ�����ʵ���Ũ��Ϊ ��
����1 molFeI2��2mol FeBr2��Һ��ͨ��3 mol Cl2����Һ�������������ӵĸ���֮���� ��
����a molFeI2��bmol FeBr2��Һ��ͨ��c mol Cl2����Ҫ��֤��Һ�к������������ӣ�c��ȡֵ��Χ�� ��
(6��)��1��2mol/L��2��Fe3+��Cl-��Br-=1:2:1��3��a<C<(1.5a+0.5b)
��

��ϰ��ϵ�д�
�����Ŀ
 �������ĵ缫��ӦΪ��M��2e����M2+ ��Y��������ϡH2SO4�У�M����ϡH2SO4�����������ֽ����Ļ��������ǿ��˳���ǣ�
�������ĵ缫��ӦΪ��M��2e����M2+ ��Y��������ϡH2SO4�У�M����ϡH2SO4�����������ֽ����Ļ��������ǿ��˳���ǣ�
 ����������Fe2(SO4)3��Һ��ȫ��Ӧ���ټ���K2Cr2O7��Һ����������������ѧ��Ӧ��SO2��2Fe3����2H2O===SO
����������Fe2(SO4)3��Һ��ȫ��Ӧ���ټ���K2Cr2O7��Һ����������������ѧ��Ӧ��SO2��2Fe3����2H2O===SO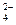 ��4H����2Fe2����
��4H����2Fe2����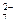 ��6Fe2����14H��===2Cr3����6Fe3����7H2O��
��6Fe2����14H��===2Cr3����6Fe3����7H2O��